 Harbour seal distribution
Harbour seal distribution
2.1 Ecological relevance
The distribution of seals reflect changes in the number of marine top predators in the Baltic Sea. Being top predators of the marine ecosystem, marine mammals are good indicators of the state of food webs, levels of hazardous substances and direct human disturbance. Seals are exposed to bottom-up effects of ecosystem changes at lower trophic levels, but also to variations in climate (length of seasons and ice conditions) and human impacts. These pressures can affect seals indirectly through e.g., decline of fish stocks, levels of harmful substances, reproductive success in addition to causing direct mortality by hunting or by-catch. The vulnerability of seals to these pressures make them good indicators for measuring the environmental status of ecosystems.
The distribution is affected by availability of suitable habitats, food and other resources, as well as anthropogenic disturbance. It is also affected by the abundance of seals. After a low phase in abundance levels recolonization of depleted areas can take time.
Regarding harbour seal, this indicator is applicable over the southwestern parts from Kattegat to Western Gotland basin.
2.2 Policy relevance
The core indicator(s) on the population trends and abundance of Baltic seals addresses the Baltic Sea Action Plan (BSAP 2021) Biodiversity segment goal of a “Baltic Sea ecosystem [that] is healthy and resilient”. The ecological objectives under this goal are also clearly relevant: ‘Viable populations of all native species’, ‘Natural distribution, occurrence and quality of habitats and associated communities’, and ‘Functional, healthy and resilient food webs’.
The HELCOM Recommendation 27/28-2 Conservation of seals in the Baltic Sea area outlines the conservation goals of seals agreed on at HELCOM. The recommendation is implemented to reach the BSAP goals. The recommendation conservation goals are used as the basis for defining this indicator’s threshold value.
The indicator also has clear relevance for the EU Marine Strategy Framework Directive (MSFD), for those Contracting Parties that are also EU Member States. In particular the relevance is high for MSFD Descriptor 1 that addresses species and habitats and also for Descriptor 4 that addresses ecosystems, including food webs.
A summary overview of policy linkages is provided in Table 1, below.
In some Contracting Parties, the indicator also has potential relevance for implementation of the EU Water Framework Directive (WFD) and Habitats Directive. The WFD includes status categories for coastal waters as well as environmental and ecological objectives. The EU Habitats Directive (European Commission 1992) specifically states that long-term management objectives should not be influenced by socio-economic considerations, although they may be considered during the implementation of management programmes provided the long-term objectives are not compromised. All seals in Europe are also listed under the EU Habitats Directive Annex II, and member countries are obliged to monitor the status of seal populations.
Table 1. Overview of policy relevance for this indicator.
| Baltic Sea Action Plan (BSAP) | Marine Strategy Framework Directive (MSFD) | |
| Fundamental link
|
Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
|
| Complementary link
|
Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
Segment: Hazardous substances and litter goal Goal: “Baltic Sea unaffected by hazardous substances and litter”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
Descriptor 4 Ecosystems, including food webs.
Descriptor 8 Concentrations of contaminants are at levels not giving rise to pollution effects.
|
| Other relevant legislation: |
|
|
Good status reflected through the distribution of seals in the Baltic Sea is based on concepts developed for the conservation of seals. The concept for defining threshold values to indicate good status is derived from the general management principle in the HELCOM Recommendation 27/28-2, which states the aim to allow breeding seals to expand to suitable breeding distribution in all regions of the Baltic Sea.
Good status is achieved when the threshold values for all considered parameters are achieved (Breeding distribution, Moulting distribution and Area of occupancy). Good status is achieved when the distributions of seals are close to pristine conditions (e.g. 100 years ago), or where appropriate when all currently available haul-out sites are occupied (modern baseline), and when no decrease in area of occupation occurs (Figure 2). Three different parameters of distribution are given for all species of seals: 1) Breeding distribution on land or ice, 2) Moulting distribution on land or ice, which refers to haulouts used for moulting and resting and 3) Area of occupancy, which includes sea areas used for transport and foraging.
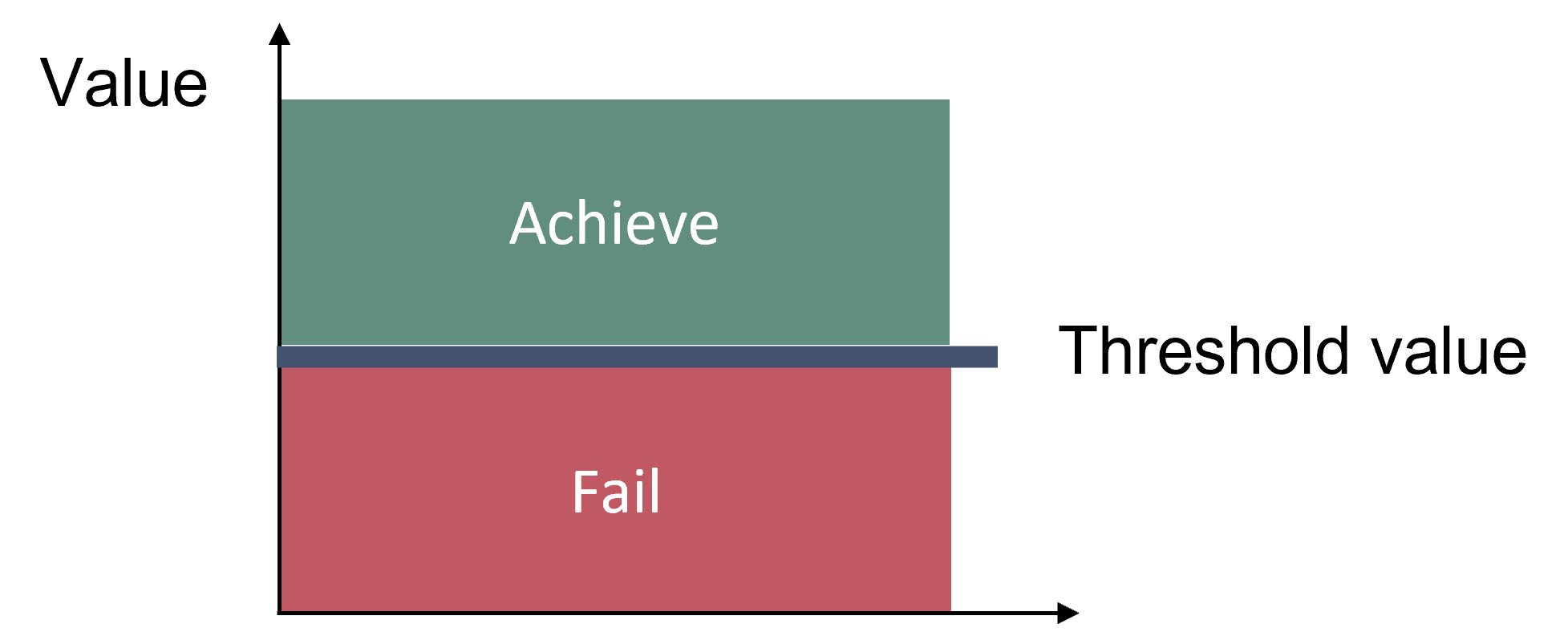
Figure 2. Good status is achieved when distribution of seals is close to pristine conditions (e.g. 100 years ago), or where appropriate when all currently available haul-out sites are occupied (modern baseline), and when no decrease in area of occupation occurs.
3.1 Setting the threshold value(s)
The following criteria are used to evaluate whether the threshold value is achieved or failed:
- Breeding distribution. The threshold value is achieved when available land breeding sites are colonized, and distribution is not diminishing.
- Moulting distribution: The distribution of haul-out sites used for resting and moulting of harbour seals are almost identical to the distribution of breeding sites. The threshold value is achieved when all existing suitable sites are colonized.
- Area of occupancy: the threshold value is achieved when seals have access to all feeding grounds and they can move freely among haul-out sites and the feeding grounds.
The modern baseline approach is applied for harbour seal distribution since some formerly used haul-out sites have disappeared in the southern Baltic as a consequence of exploitation of sand for industrial use. This type of a modern baseline should be defined so that the species will thrive and persist in the future.
4.1 Status evaluation
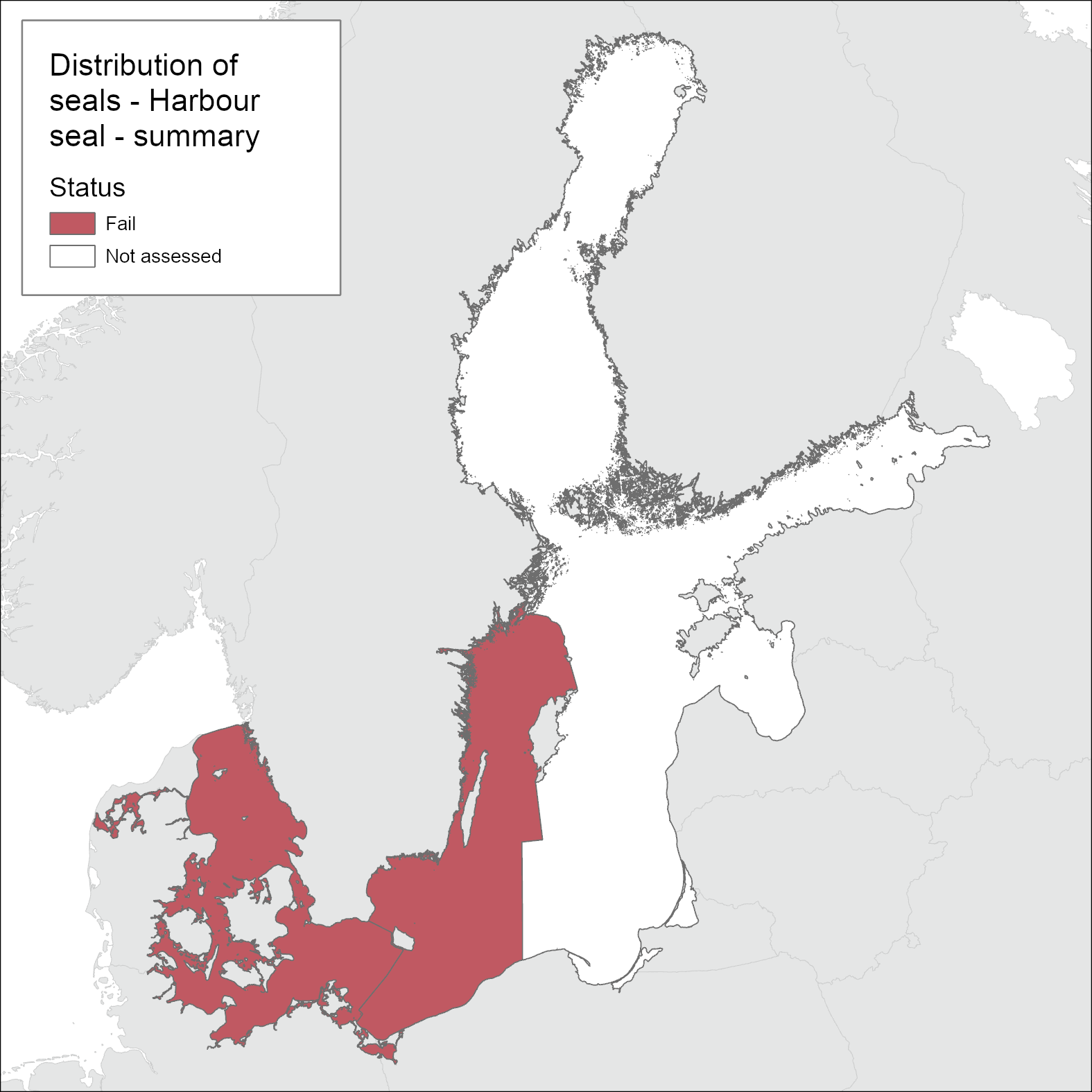
For the distribution indicator, the harbour seals subpopulations Kalmarsund (Bornholm Basin and Western Gotland Basin), SW Baltic (Arkona basin, Bay of Mecklenburg, Kiel Bay, Great Belt and the Sound), Kattegat and Limfjord are assessed independently as two separate management units (as set out under HELCOM Recommendation 27/28-2; 1) Harbour seals in the Kalmarsund region (Sweden); 2) Southwestern Baltic and Kattegat harbour seals (Denmark, Germany, Poland, Sweden)) using distribution during pristine conditions as the base-line (Figure 1). Additional information is also provided at smaller scales to represent recent advances to gain new knowledge on seal distribution.
Moulting and breeding distribution:
In the areas of the Kattegat and Limfjord the harbour seal populations are observed at all historical haul-out sites during the moulting survey (Figure 3). In the SW Baltic, harbour seals do not currently breed regularly at historical localities south of the island of Fyn or in the Great Belt. There is also a lack of occupied haul-out sites along the German coast. In the Kalmarsund, harbour seals are increasing and showing signs of expanding and still colonizing new areas. Haul-out sites are only routinely monitored across the range during the moulting-time in August. Thus, the evaluation is most accurate in determining moulting distribution. However, harbour seals are relatively sedentary compared to the other Baltic Sea seal species and their moulting haul-out sites mostly correspond to their pupping sites. As such, the Limfjord and the Kattegat, if assessed separately would achieve good environmental status, whilst the Kalmarsund and SW Baltic fail to achieve good environmental status for the moulting and distribution assessment (Figure 4). When grouped only as the agreed management units however, both fail to achieve good environmental status.
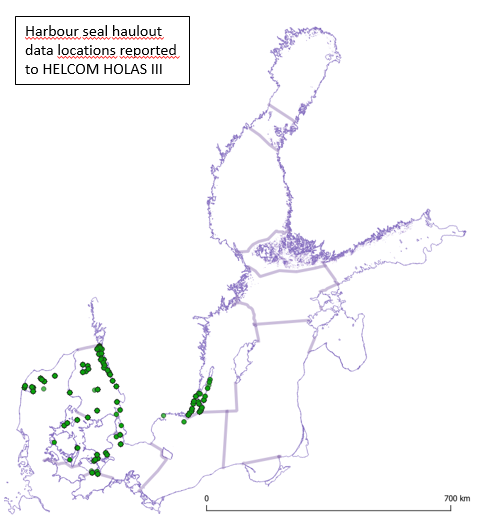
Figure 3: Distribution of harbour seal moulting haulouts in the Baltic Sea according to the data reported to HELCOM HOLAS III. The map includes all currently known haul-out sites. Harbour seal moult haulouts correspond to their breeding haulouts. Harbour seals have not yet recolonized all the historically known and available breeding and moulting haul-out sites in southwestern Baltic and in Kalmarsund.
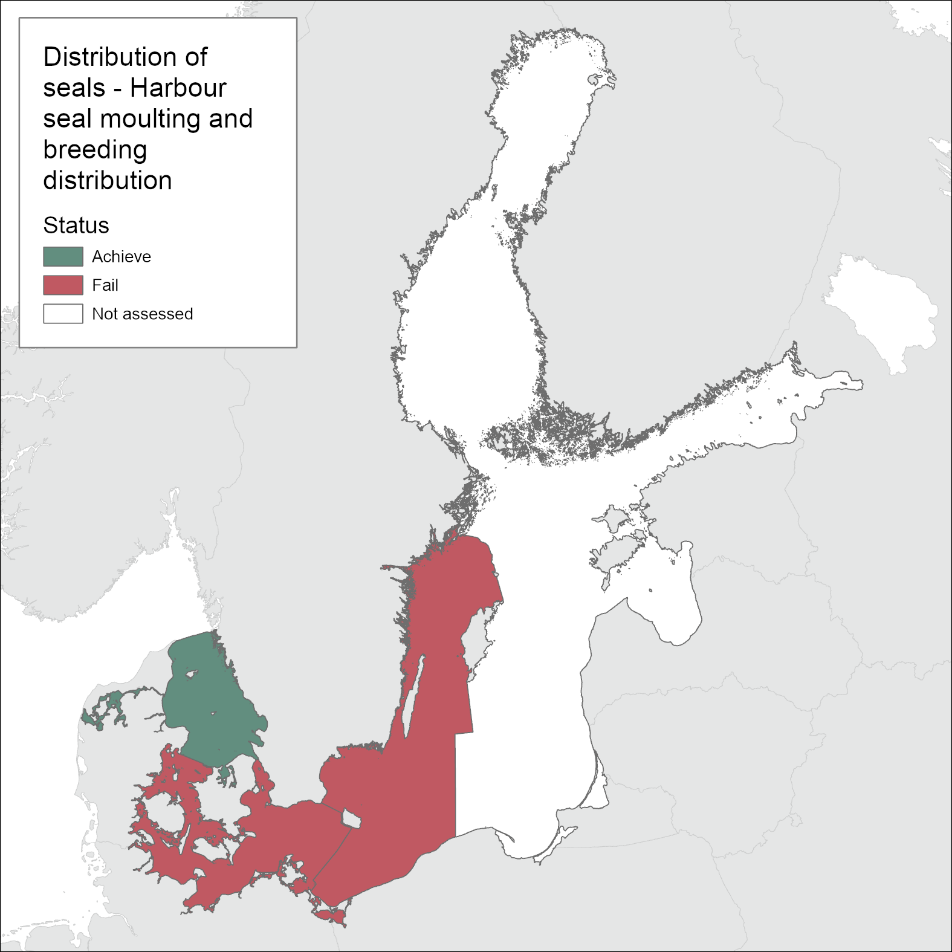
Figure 4: Evaluation of harbour seal moulting and breeding distribution.
Area of occupancy:
Although there is no structured monitoring of at sea occupancy harbour seal behaviour and movement at sea have been studied with help of satellite tracking devices. Harbour seals have been tagged to study movements in Kattegat, Limfjord and in the SW Baltic. Based on these data it has been evident that seals can travel freely among sites and feeding grounds. There have been no tagging efforts in the Kalmarsund area, but there is also no evidence to suggest that movement is restricted as the population appears to expand is distribution as observed by the occupancy of new haul-out sited during moulting. As such, harbour seals achieve good environmental status, for all subpopulations, for area of occupancy evaluation.
Overall evaluation of Distribution of Baltic harbour seals:
Based on one-out-all-out concept, the Distribution indicator achieves good status in Limfjord and Kattegat. In SW Baltic and Kalmarsund the breeding and moulting distributions have not yet reached the pristine levels leading the Distribution indicator fail to achieve good status. However, when evaluated only at the scale of the two agreed management areas both of these areas fail to achieve Good Environmental Status (Figure 1).
4.2 Trends
Changes from the previous evaluation of the status of harbour seal distribution are mainly due to new information considered.
In HOLAS II, Kalmarsund population was assessed as having occupied all of its pristine distribution. However, in that evaluation, it was not considered that the species has been present for example at Gotland and northern part of Kalmarsund (Härkönen et al. 2005). During the current assessment period Kalmarsund harbour seals have shown signs of expanding to new suitable haulouts towards the north and possibly the west. Understanding that our knowledge on the exact historical haulouts is still incomplete, we re-evaluated the pristine distribution to be larger than the current distribution. This led to a conclusion in HOLAS III that the threshold for breeding and moulting distribution has not yet been achieved.
Recolonization of historical haulouts has been observed in SW Baltic, too. After reports of increasing seal occurrence in the South Funen Archipelago in recent years, a pilot survey was conducted during the moulting season of 2021, revealing that the haul-outs in this area under a modern baseline were occupied after local extinction in the early 1900s. Data on breeding distribution in this area were not available for the current evaluation. Former haul-out range along the German Baltic coast has not been reoccupied, even under a modern baseline, disregarding previous haul-outs that are now permanently lost.
4.3 Discussion
An overview of the status evaluation and a comparison between the current (HOLAS 3, 2016-2021) and previous (HOLAS II, 2011-2016) periods is provide below in Table 2.
Table 2. Overview of status evaluation and comparison between assessment periods.
| HELCOM Harbour seal management unit |
Threshold value achieved/failed – HOLAS 2 |
Threshold value achieved/failed – HOLAS 3 |
Distinct trend between current and previous evaluation. | Description of outcomes, if pertinent. |
| Evaluation based on two currently agreed management areas | ||||
| Southwestern Baltic and Kattegat harbour seals | Not assessed in same format | Failed, see below. | NA, in the previous assessment period the evaluation was applied in an alternative format so no direct comparison can be carried out. | Good status is not achieved due to the failure to achieve the threshold value for moulting and breeding distribution in the SW Baltic area despite all other parameters being achieved elsewhere in this management area. |
| Kalmarsund | Achieved | Failed | Distribution is showing signs of increase, but the extent of the pristine distribution, which is the threshold for good status, was re-evaluated due to the observed increases. The switch in status from achieve to fail is therefore not as simple to interpret as a deterioration since increasing distribution is a positive sign for the population. | GES is not achieved in the current period and this is dominantly driven by an increase in distribution indicative of the fact that the historic range appears still not to have been achieved. |
| Smaller scale of evaluation additionally applied for contextual information | ||||
| Kattegat | Achieved | Achieved | Stable, no change in status has occurred and all parameters remain above their respective threshold values. | All parameters utilised to carry out the evaluation achieve the threshold values and thus GES is achieved. |
| Limfjord | Achieved | Achieved | Stable, no change in status has occurred and all parameters remain above their respective threshold values. | All parameters utilised to carry out the evaluation achieve the threshold values and thus GES is achieved. |
| SW Baltic | Failed | Failed | Stable, no change in status has occurred and the failure to achieve the threshold value for moulting and breeding distribution remains the common thread. | Good status is not achieved due to the failure to achieve the threshold value for moulting and breeding distribution. |
Changes to the way in which evaluations were applied between the two assessment periods compared result in some different comparisons, however, equivalent smaller scale evaluations are also applied in HOLAS 3 to allow better comparison. In addition, see future work, there isa need to re-evaluate the management areas applied for this species as recent scientific advances suggest a finer scale of evaluation and management is required. The ‘deterioration’ apparent in status for the Kalmarsund population must also be clarified carefully as the status change is due to an expansion of the population to areas previously believed to be outside of its natural historic range, thus such changes can not per se be seen as a deterioration.
The confidence for harbour seal moult distribution is considered to be high in most assessment units, as moult counts are currently carried out at a high spatial and temporal frequency. Surveys in the South Funen Archipelago, where harbour seals were locally extinct by the early 1900s are only available for 2021 and no data on breeding distribution are available for this area for the current evaluation. Confidence for breeding distribution is moderate or low in Sweden where pup counts are not carried out regularly. There, observations on breeding sites are sporadic, but supporting the expectation that the breeding sites are largely the same as moulting sites. Understanding on the area of occupancy is based on some telemetry studies showing the extent of harbour seals’ foraging area around their haulouts. Such telemetry studies are lacking for the Kalmarsund population. However, the threshold for area of occupancy is the free access for seals to use their haulouts and foraging grounds. For that, no obstructions are known.
Historically, hunting of seals has been a major human pressure on all the seal species in the Baltic Sea. A coordinated international campaign was initiated in the beginning of the 20th century with the aim of exterminating the seals (Anon. 1895). Bounty systems were introduced in Denmark, Finland and Sweden over the period 1889-1912, and very detailed bounty statistics provide detailed information on the hunting pressure. The original population sizes were about 180,000 for ringed seals, 80,000 for Baltic grey seals and 5,000 for the Kalmarsund population of harbour seals (Harding & Härkönen 1999; Härkönen & Isakson 2011). Similar data from the Kattegat and Skagerrak suggest that populations of harbour seals amounted to more than 17,000 seals in this area (Heide-Jørgensen & Härkönen 1988).
Table 3 Brief summary of relevant pressures and activities with relevance to the indicator.
| General | MSFD Annex III, Table 2a | |
| Strong link | The main pressures affecting the distribution of Baltic seal populations include hunting, by-catches, disturbance and destruction of haul-out sites. | Biological
|
| Weak link | The effects of climate change are a threat to the ringed seal that breeds on sea ice.
Fishery and food availability. |
Substances, litter and energy
|
The hunting pressure resulted in extirpation of grey and harbour seals in Germany and Poland in 1912, and grey seals were also extirpated from the Kattegat by the 1930s. Ringed seals declined to about 25,000 seals in the 1940s, whereas grey seals were reduced to about 20,000 (Harding & Härkönen 1999) over the same time period. Ringed seal breeding occurred in Stockholm county up to the beginning of the 1940s, but ceased in the mid of that decade (Hult 1943). A similar rate of reduction of harbour seals occurred in the Kalmarsund and the Kattegat (Heide-Jørgensen & Härkönen 1988; Härkönen & Isakson 2011). However, after these heavy reductions, populations appear to have been stable up to the 1960s (Harding & Härkönen 1999).
Climate change is expected to have significant impacts on the Baltic Sea ecosystem (HELCOM and Baltic Earth, 2021). Climate change will likely have widespread impacts on the Baltic Sea ecosystem, including on higher trophic levels. Such changes may influence status evaluations and also need to be reflected in management (e.g. potentially the need to be precautionary). Climate change impacts could include flooding of haul out sites, changed temperature, stratification, and altered prey distribution, quality and quantity, all of which, though difficult to current predict risk impacts on marine mammals. Being at the top of the marine food web, these predators are sensitive to changes throughout the ecosystem, and changes in food webs on which they rely (and for which our current understanding is poor) may be significant with potential changes in food availability and altered transfer of contaminants.
Such food web and ecosystem changes may force a re-distribution of seals but a significant direct impact is the projected sea level rise which would flood many or all harbour seal haulouts in the SW Baltic (Meier et al. 2022). However, the effects of climate change should themselves not be directly included in evaluations according to the Habitat Directive.
Harbour seal distribution achieves the good status in Kattegat and Limfjord areas, if assessed independently, where all available haulouts for breeding, moulting and resting are occupied and they have free access to and between these sites and foraging grounds. However in the SW Baltic area the status is not good, based on harbour seals not regularly occurring at historical localities south of the island of Fyn or in the Great Belt.Thus when assessed as a single management area the overall status fails to achieve GES. In the Kalmarsund sub-population (management area) harbour seals have not yet colonized all available and suitable haulouts for breeding, moulting and resting and therefore do not achieve good environmental status.
8.1 Future work or improvements needed.
Pup counts covering the whole breeding distribution would improve the geographical and temporal resolution of the breeding data. Regular telemetry studies in all populations would provide more accurate information on the foraging grounds and movement behaviour as well as potential changes in them. The proposed approach to re-evaluate harbour seal management units, as set out in intersessional work under EG MAMA and State and Conservation (i.e. developing of more and smaller relevant management areas based on latest science and re-evaluating relevant Limit Reference Levels), needs to be carried out to improve future evaluations.
9.1 Scale of assessment
This core indicator evaluates the distribution of Baltic Sea seal species using HELCOM assessment unit scale 2 (division of the Baltic Sea into 17 sub-basins), aggregated into the two management areas defined under HELCOM Recommendation 27/28-2 Conservation of seals in the Baltic Sea area. The assessment units are defined in the HELCOM Monitoring and Assessment Strategy Annex 4.
The existing management plans for seals operate according to management units that are based on the distribution of seal populations. The management units typically encompass a handful of HELCOM scale 2 assessment units. Evaluations are therefore done by grouping HELCOM assessment units to align with the management units defined for each seal population.
- Harbour seals in the Kalmarsund, Sweden, constitute a separate management unit within Bornholm and Western Gotland Basins.
Harbour seals in the southwestern Baltic occur in Arkona Basin, Bay of Mecklenburg, Kiel Bay, southern part of Great Belt and The Sound, the Kattegat population of harbour seals inhabits Kattegat and northern part of Great Belt, and the Harbour seals in the Limfjord form a separate management unit and are genetically distinct from the Kattegat harbour seals (Olsen et al. 2014).
This second management unit is the one requiring revision based on recent scientific studies and greater details on the sub-sections of it are provided in the current results.
9.2 Methodology applied
Monitoring methodology:
HELCOM common monitoring relevant for the seal population trends is documented on a general level in the HELCOM Monitoring Manual under the sub-programme: Seal abundance.
HELCOM monitoring guidelines for seals were adopted in 2014 and updated in 2018 (HELCOM Guidelines for monitoring seal abundance and distribution (2018).
The three regularly occurring seal species in the Baltic Sea: harbour seal, ringed seal and grey seal, are monitored at their haul-outs on land during their annual moulting and pupping seasons, with the aim of estimating the abundance and trends (moulting counts) and pup production (pupping counts). Ringed seals are counted during moult on the ice. Where possible, the monitoring is performed using aerial surveys, where the seal haul-outs are photographed during the relevant periods in areas where there is a significant occurrence of seals.
Detailed descriptions of the survey methodology and analysis of results are given in the HELCOM monitoring guidelines (HELCOM Guidelines for monitoring seal abundance and distribution (2018).). The monitoring carried out according to these guidelines will not be very sensitive to detecting positive changes in range and mainly constriction in range can be detected. Other means are needed for detecting range expansion, and surveys are adjusted to cover expansions in range based on satellite telemetry data and other observations.
Current monitoring:
The monitoring activities relevant to the indicators that are currently carried out by HELCOM Contracting Parties are described in the HELCOM Monitoring Manual in the Monitoring Concept Table.
Sub-programme: Seal Abundance
Monitoring Concept Table
Current monitoring covers all haul-out sites presently used by seals in the Baltic Sea and is considered to be sufficient to cover the needs of the indicator except for southern ringed seals. See description in the Assessment Requirements of the HELCOM Monitoring Manual.
10 Data
The data and resulting data products (e.g. tables, figures and maps) available on the indicator web page can be used freely given that it is used appropriately and the source is cited as following:
HELCOM (2023) Distribution of Baltic seals. HELCOM core indicator report. Online. [Date Viewed], [Web link]. ISSN 2343-2543.
Result: Distribution of Baltic seals – Harbour seal
Data: Distribution of Baltic seals – Harbour seal
The national survey data is compiled annually by the HELCOM Seal Expert Group. A regional database has been developed and is hosted at the HELCOM Secretariat. The new database will include detailed spatial information and is to be updated annually prior to HELCOM Seal Expert Group meetings.
Status evaluations are to be accomplished by the Lead and co-Lead countries. The outcome of such assessments will be presented and discussed at future HELCOM Seal Expert Group meetings.
The first compilations for the database have been completed and an intermediate version of the seal database can be accessed. During 2015-2016 work continued to operationalize the database. Further metadata was included at a later stage.
The data collected and used in the indicator are based on national aerial surveys. The survey methodology is described in the relevant HELCOM Guidelines for monitoring seal abundance and distribution (2018). This data covers only haul-out sites and not areas used e.g. as foraging grounds.
This indicator report for HOLAS 3 was prepared by Markus Ahola, Anders Galatius and Anja Carlsson.
The assessment principles, methodology and background information are largely based on the previous assessment report by Tero Härkönen, Anders Galatius, Karin Hårding, Olle Karlsson, Markus Ahola, Morten Tange Olsen.
HELCOM Expert Group on Marine Mammals (EG MAMA)
HELCOM Secretariat: Jannica Haldin, Florent Nicolas, Petra Kääriä, Owen Rowe.
This version of the HELCOM core indicator report was published in April 2023:
The current version of this indicator (including as a PDF) can be found on the HELCOM indicator web page.
Earlier versions of this indicator are available at:
Distribution of Baltic seals HELCOM core indicator 2018 (pdf)
HOLAS II component – core indicator report July 2017 (pdf)
Population growth rate, abundance and distribution of marine mammals 2013 (pdf)
Anon (1895) Svensk fiskeritidskrift 1895.
Bäcklin, B.-M., Moraeus, C., Roos, A., Eklöf, E., Lind, Y. (2011) Health and age and sex distributions of Baltic grey seals (Halichoerus grypus) collected from bycatch and hunt in the Gulf of Bothnia. ICES Journal of Marine Science, 68: 183–188.
Bäcklin, B.-M., Moraeus, C., Kauhala, K., Isomursu. M. (2013) Pregnancy rates of the marine mammals – Particular emphasis on Baltic grey and ringed seals. HELCOM web portal.
Bergman, A., Olsson, M. (1985) Pathology of Baltic grey seal and ringed seal females with special reference to adrenocortical hyperplasia: Is environmental pollution the cause of a widely distributed disease syndrome. Finnish Game Res 44: 47-62.
European Commission (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora (Habitats Directive). Off. J. Eur. Union 206: 7–50.
European Commission (2008) Directive 2008/56/EC of the European Parliament and the Council establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union L 164: 19-40.
European Commission (2010) Commission Decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters (2010/477/EU). Off. J. Eur. Union L232: 12-24.
European Commission (2017) Commission Decision of (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment, and repealing Decision 2010/477/EU. May 2017.
Galatius, A., Ahola, M., Härkönen, T., Jüssi, I., Jüssi, M., Karlsson, O., Verevkin, M. (2014) Guidelines for seal abundance monitoring in the HELCOM area 2014. Available at: http://helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Guidelines%20for%20Seal%20Abundance%20Monitoring%20HELCOM%202014.pdf
Goodman, S.J. (1998) Patterns of extensive genetic differentiation and variation among European harbor seals (Phoca vitulina vitulina) revealed using microsatellite DNA polymorphisms. Molecular Biology and Evolution 15(2): 104-118.
Fietz, K., A. Galatius, J. Teilmann, R. Dietz, A. K. Frie, A. Klimova, P. Palsbøll, L. Jensen, J. A. Graves, J. I. Hoffman and M. T. Olsen (2016). “Shift of grey seal subspecies boundaries in response to climate, culling and conservation.” Molecular Ecology 25(17): 4097-4112.
Harding, K.C., Härkönen, T.J. (1999) Development in the Baltic grey seal (Halichoerus grypus) and ringed seal (Phoca hispida) populations during the 20th century. Ambio 28: 619-627.
Härkönen, T., Lunneryd, S.G. (1992) Estimating abundance of ringed seals in the Bothnian Bay. Ambio 21:497-510.
Härkönen, T., Stenman, O., Jüssi, M., Jüssi, I., Sagitov, R., Verevkin, M. (1998) Population size and distribution of the Baltic ringed seal (Phoca hispida botnica). In: Ringed Seals (Phoca hispida) in the North Atlantic. Edited by C. Lydersen and M.P. Heide-Jørgensen. NAMMCO Scientific Publications Vol. 1: 167-180.
Härkönen, T., Harding, K.C., Goodman, S., Johannesson, K. (2005) Colonization history of the Baltic harbor seals: Integrating archaeological, behavioural and genetic data. Marine Mammal Science 21: 695-716.
Härkönen, T., Brasseur, S., Teilmann, J., Vincent, C., Dietz, R., Reijnders, P., Abt, K. (2007) Status of grey seals along mainland Europe, from the Baltic to France. NAMMCO Scientific Publications 6: 57-68.
Härkönen, T., Harding, K., Rasmussen, T.D., Teilmann, J., Dietz, R. (2007) Age- and Sex-specific Mortality Patterns in an Emerging Wildlife Epidemic: the Phocine Distemper in European Harbour Seals. PLoS ONE 2(9): e887. doi: 10.1371/journal.pone.0000887
Harkonen, T., Bäcklin, B.-M., Barrett, T., Bergman, A., Corteyn, M., Dietz, R., Harding, K., Malmsten, J., Roos, A., Teilmann, T. (2008) Mass mortality in harbour seals and harbour porpoises caused by an unknown pathogen. The Veterinary Record 162: 555-556.
Harkonen, T., Jüssi, M., Jüssi, I., Verevkin, M., Dmitrieva, L., Helle, E., Sagitov, R., Harding, K.C. (2008) Seasonal activity budget of adult Baltic ringed seals (Phoca hispida botnica). PLoS ONE 3(4): e2006.doi:10.1371/journal.pone.0002006
Harkonen, T., Isakson, E. (2011) Historical and current status of harbour seals in the Baltic proper. NAMMCO Scientific Publications 8: 71-76.
Heide-Jørgensen, M.-P., Härkönen, T. (1988) Rebuilding seal stocks in the Kattegat-Skagerrak. Marine Mammal Science 4(3): 231-246.
Helle, E. (1980) Lowered reproductive capacity in female ringed seals (Pusa hispida) in the Bothnian Bay, northern Baltic Sea, with special reference to uterine occlusions. Annales Zoologica Fennici 17: 147-158.
Hult, J. (1943) Sälen och säljakten i Östersjön under de senaste decennierna. Svenska Jägereförbundets tidskrift 81: 365-373.
Jüssi, M., Härkönen, T., Jüssi, I., Helle, E. (2008) Decreasing ice coverage will reduce the reproductive success of Baltic grey seal (Halichoerus grypus) females. Ambio 37: 80–85.
Meier, H.E.M., Döscher, R., Halkka, A. (2004) Simulated distributions of Baltic Sea ice in the warming climate and consequences for the winter habitat of the Baltic Ringed Seal. AMBIO 33: 249–256.
Meier, H. E. M., Kniebusch, M., Dieterich, C., Gröger, M., Zorita, E., Elmgren, R., Myrberg, K., Ahola, M., Bartosova, A., Bonsdorff, E., Börgel, F., Capell, R., Carlén, I., Carlund, T., Carstensen, J., Christensen, O. B., Dierschke, V., Frauen, C., Frederiksen, M., Gaget, E., Galatius, A., Haapala, J. J., Halkka, A., Hugelius, G., Hünicke, B., Jaagus, J., Jüssi, M., Käyhkö, J., Kirchner, N., Kjellström, E., Kulinski, K., Lehmann, A., Lindström, G., May, W., Miller, P., Mohrholz, V., Müller-Karulis, B., Pavón-Jordán, D., Quante, M., Reckermann, M., Rutgersson, A., Savchuk, O. P., Stendel, M., Tuomi, L., Viitasalo, M., Weisse, R., and Zhang, W. 2021. Climate Change in the Baltic Sea Region: A Summary, Earth Syst. Dynam. 13: 457-593, https://doi.org/10.5194/esd-13-457-2022.
Oksanen, S.M., Niemi, M., Ahola, M.P., Kunnasranta, M. (2015) Identifying foraging habitats of Baltic ringed seals using movement data. Movement Ecology DOI 10.1186/540462:015-0058-1.
Olofsson, O. (1933) Om vikaresälens hispida annelata storlek och föda mm. Fauna och Flora 1933: 17-28.
Olsen, M.T., Wesley Andersen, L., Dietz, R., Teilmann, J. Harkonen, T., Siegismund, H.R. (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology 23: 815-831.
Sundqvist, L., Harkonen, T. Svensson, C.J., Harding, K.C. (2012) Linking climate trends to population dynamics in the Baltic ringed seal – Impacts of historical and future winter temperatures. Ambio. DOI 10.1007/s13280-012-0334-x
Teilmann, J., Riget, F., Harkonen, T. (2010) Optimising survey design in Scandinavian harbour seals: Population trend as an ecological quality element. ICES Journal of Marine Science 67: 952–958.
Bergman, A. (1999) Health condition of the Baltic grey seal (Halichoerus grypus) during two decades. Apmis 107(1‐6): 270-282.
Bigg, M.A. (1969) The harbour seal in British Columbia (No. 172). Fisheries Research Board of Canada.
Boulva, J., McLaren, I.A. (1979) Biology of the harbor seal, Phoca vitulina, in eastern Canada. Fisheries Research Board of Canada.
Caswell, H. (2001) Matrix population models: Construction, analysis, and interpretation. Second edition. Sinauer, Sunderland, Massachusetts, USA.
Dietz, R., Heide-Jørgensen, M.-P., Härkönen, T. (1989) Mass deaths of harbour seals Phoca vitulina in Europe. Ambio 18(5): 258-264.
Harding, K.C. Härkönen, T., Caswell, H. (2002) The 2002 European seal plague: epidemiology and population consequences. Ecology Letters 5: 727-732.
Harding, K.C., Härkönen, T., Pineda, J. (2003) Estimating quasi-extinction risk of European harbour seals: a reply to Lonergan and Harwood. Ecology Letters 6: 894-897.
Harding, K.C., Härkönen, T., Helander, B., Karlsson, O. (2007) Status of Baltic grey seals: Population assessment and risk analysis. NAMMCO Scientific Publications 6: 33-56.
Härkönen, T. Heide-Jørgensen, M.-P. (1990) Density and distribution of the ringed seal in the Bothnian Bay. Holarctic Ecology 13(2): 122-129.
Härkönen, T., Harding, K.C. (2001) Spatial structure of harbour seal populations and the implications thereof. Can. J. Zool. 79: 2115-2127.
Härkönen, T., Dietz, R., Reijnders, P., Teilmann, J., Harding, K., Hall, A., Brasseur, S., Siebert, U., Goodman, S., Jepson, P., Dau Rasmussen, T., Thompson, P. (2006) A review of the 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Diseases of Aquatic Organisms 68: 115-130.
Harkonen, T., Harding, K.C. (2011) Predicting recurrent PDV epidemics in European harbour seals. NAMMCO Scientific Publications 8: 275-284
Harwood, J., Prime, J.H. (1978) Some factors affecting the size of British grey seal populations. Journal of Applied Ecology: 401-411.
Heide-Jørgensen, M.-P., Härkönen, T. (1992) Epizootiology of seal disease. Journal of Applied Ecology 29: 99-107.
Heide-Jørgensen, M.-P., Härkönen, T. Dietz, R., Thompson, P. (1992) Retrospective of the 1988 European seal epizootic. Diseases of Aquatic Organisms 13: 37-62.
Hiby, L., Lundberg, T. Karlsson, O. Watkins, J., Jüssi, M., Jüssi, I., Helander, B. (2007) Estimates of the size of the Baltic grey seal population based on photo-identification data. NAMMCO Scientific Publications [S.l.] (6): 163-175. Oct. 2013. ISSN 2309-2491. Available at: <http://septentrio.uit.no/index.php/NAMMCOSP/article/view/2731>. doi:http://dx.doi.org/10.7557/3.2731
Karlsson, O., Härkönen, T., Bäcklin, B.M. (2008) Populationer på tillväxt. Havet 2008: 91-92.
Kokko, H., Helle, E. J., Ranta, E., Sipilä, T. (1999) Backcasting population sizes of ringed and grey seals in the Baltic and Lake Saimaa during the 20th century. Annales Zoologici Fennici 36: 65-73.
Mortensen, P., Bergman, A., Bignert, A., Hansen, H.J., Härkönen, T., Olsson, M. (1992) Prevalence of skull lesions in harbour seals Phoca vitulina in Swedish and Danish museum collections during the period 1835-1988. Ambio 21: 520-524.
Olsen, M.T., Andersen, S.M., Teilmann, J., Dietz, R., Harkonen, T. (2010) Status of the harbour seal in Southern Scandinavia. NAMMCO Scientific Publications 8: 77-94.
Palo, J.U., Mäkinen, H.S., Helle, E., Stenman, O., Väinölä, R. (2001) Microsatellite variation in ringed seals (Phoca hispida): genetic structure and history of the Baltic Sea population. Heredity 86: 609–617. doi: 10.1046/j.1365-2540.2001.00859.x.
Sipilä (2003) Conservation biology of Saimaa ringed seal (Phoca hispida saimensis) with reference to other European seal populations. PhD Thesis. http://ethesis.helsinki.fi/julkaisut/mat/ekolo/vk/sipila/conserva.pdf?q=phoca
Stenman, O., Halkka, A., Helle, E., Keränen, S., Nummelin, J., Soikkeli, M., … Tanskanen, A. (2005) Numbers and occurrence of grey seals in the Finnish sea area in the years 1970-2004. In Symposium on Biology and Management of Seals in the Baltic area. Kala-ja riistaraportteja nro (346): 58-61.
Svensson, C.J., Hansson, A. Harkonen, T., Harding, K. (2011) Detecting density dependence in growing seal populations. AMBIO (2011) 40: 52–59. doi 10.1007/s13280-010-0091-7
Vanhatalo, J., Vetemaa, M., Herrero, A., Aho, T., Tiilikainen, R. (2014) By-Catch of Grey Seals (Halichoerus grypus) in Baltic.