Nutritional status of seals
Nutritional status of seals
2.1 Ecological relevance
Marine mammals are top predators in the marine ecosystem and therefore good indicators of changes in biotic and abiotic environment, for example variation in food webs due to fishing. Marine mammals accumulate hazardous substances such as heavy metals and PCBs in their tissues (so-called bioaccumulation) and thus reflect the level of pollution in the environment. Seals are also affected by human disturbances such as hunting, fishing and pollution (e.g. chemical and noise pollution), as well as infectious diseases and climate change.
Blubber acts as the energy storage of seals and thus a reduction in blubber affects reproduction and survival of individual seals and is an early warning of decline in population trends, as confirmed by multiple scientific studies worldwide. However, blubber thickness responds to short-term variations in the environment and is a versatile indicator that complements the population trend and reproductive rate indicators.
The three seal species included in this indicator are all phocid seals that have a life history where they rely on stored fat reserves for over-winter survival and reproduction. Their pups are nursed during a few weeks in the spring (grey and ringed seals) or summer (harbour seals) and female weight loss during this short period is massive, up to 30-50% of total body weight (Kovacs & Lavigne 1986; McCann et al. 1989; Haller et al. 1996). During summer and autumn, seals build up their fat reserves (Nilssen et al. 2001; Hauksson & Bogason 1997). Failure to reach critical fat reserves in late autumn may result in decreased survival. A study on individually marked harbour seals also showed that winter survival in the young of the year was highly dependent on the autumn weight (Härkönen & Harding 2001, Harding et al. 2005). The range in survival rate was large, from 96% in well-fed pups to only 65% in lean pups. Similar fluctuations in life history parameters have also been observed for example inharp seals, Canadian harbour seals and ringed seals (Harwood & Prime 1978; Fowler 1981; Kjellqwist et al. 1995; Krafft et al. 2006). Thus, food abundance/quality and other factors that influence feeding success are important. Blubber thickness is one vital component indicative of nutritional status and is most informative during late autumn and winter as it is at its annual maximum.
2.2 Policy relevance
The core indicator on nutritional status of seals addresses the 2021 Baltic Sea Action Plan’s (BSAP) Biodiversity segment with the ecological objectives “Viable populations of all native species“, “Natural distribution, occurrence and quality of habitats and associated communities” and “Functional, healthy and resilient food webs”. The core indicator is relevant to the following specific BSAP action:
- B19: By 2023 finalise and implement national or local conservation and/or management plans for grey seals.
- B20 By 2023 finalise and implement national conservation and/or management plans for ringed seals.
- B21 By 2025 protect the ringed seal in the Gulf of Finland, including to significantly reduce by-catch and to improve the understanding of the other direct threats on the seals, and urge transboundary co-operation between Estonia, Finland and Russia to support achieving a viable population of ringed seals in the Gulf.
- ‘B23: By 2025 develop, and by 2027 implement, and enforce compliance with ecologically relevant conservation plans or other relevant programmes or measures, limiting direct and indirect pressures stemming from human activities for threatened and declining species. These will include joint or regionally agreed conservation measures for migrating species.
The HELCOM Recommendation 27/28-2 ‘Conservation of seals in the Baltic Sea area’ outlines the conservation goals, which the indicator’s threshold value is based on. The explicit long-term objectives of management plans to be elaborated are: Natural Abundance, Natural Distribution, and a health status that ensures the persistence of marine mammals in the Baltic Sea.
The core indicator also addresses the following qualitative descriptors of the MSFD for determining good environmental status (European Commission 2008):
Descriptor 1: ‘Biological diversity is maintained. The quality and occurrence of habitats and the distribution and abundance of species are in line with prevailing physiographic, geographic and climatic conditions’ and
Descriptor 4: ‘All elements of the marine food webs, to the extent that they are known, occur at normal abundance and diversity and levels capable of ensuring the long-term abundance of the species and the retention of their full reproductive capacity’.
Descriptor 8: ‘Concentrations of contaminants are at levels not giving rise to pollution effects’
and the following criteria of the draft Commission Decision on GES criteria (European Commission 2016):
- D1C3 Population demographic characteristics of the species
- D1C2: The population abundance of the species
- D1C4: The species distributional range
- D4C4: Productivity of the trophic guild
- D8C2: The health of species and the condition of habitats are not adversely affected due to contaminants
Marine mammals were recognized by the MSFD Task Group 1 as a group to be assessed.
In some Contracting Parties, the indicator also has potential relevance for implementation of the EU Water Framework Directive (WFD, Chemical quality) and Habitats Directive. The WFD includes status categories for coastal waters as well as environmental and ecological objectives, whereas the EU Habitats Directive (European Commission 1992) specifically states that long-term management objectives should not be influenced by socio-economic considerations, although they may be considered during the implementation of management programmes provided the long-term objectives are not compromised. All seals species in Europe are also listed under the EU Habitats Directive Annex II (European Commission 1992), and Member States are obliged to monitor the status of seal populations.
The indicator is also relevant for the implementation of SDG 14.
Table 1. Policy relevance of indicator
| Baltic Sea Action Plan (BSAP) | Marine Strategy Framework Directive (MSFD) | |
| Fundamental link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
|
| Complementary link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
Segment: Hazardous substances and litter goal Goal: “Baltic Sea unaffected by hazardous substances and litter”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
Descriptor 4 Ecosystems, including food webs.
Descriptor 8 Concentrations of contaminants are at levels not giving rise to pollution effects.
|
| Other relevant legislation: | In some Contracting Parties also EU Water Framework Directive – Chemical quality, Habitats Directive | |
2.3. Relevance for other assessments
The status of biodiversity is assessed under the HELCOM Biodiversity integrated assessment tool (BEAT) using several core indicators. Each indicator focuses on one important aspect of the complex issue. The results are utilised in BEAT to support an overall evaluation of marine mammals.
For the grey seal, good status is achieved when blubber thickness of sub-adults is at least 40 mm for hunted seals and 35 mm for by-caught seals (HELCOM HOD 48-2015, outcome para 3.63, Annex 4). A provisional threshold value of 25 mm has been proposed if the population is assessed to be at carrying capacity (Table 2) as this presumably reflects the level where depleted fat reserves result in interference with thermoregulatory processes (HELCOM 2018).
The concept for defining a threshold value for nutritional status of seals is derived from the general management principle in the HELCOM Recommendation 27/28-2, which states that the population size is to be managed with the long-term objective of allowing seal populations to recover towards carrying capacity levels. The Recommendation further states that the long-term goal is to reach a health status that ensures the future persistence of marine mammals in the Baltic Sea.
Threshold values are established for two scenarios: for populations undergoing exponential growth and for populations at carrying capacity (Table 2).
Table 2. Threshold values set for grey seals applicable in the entire Baltic Sea as agreed by HELCOM HOD 48-2015 (outcome para 3.63, Annex 4).
| Threshold value | ||
| Samples from | Populations undergoing exponential growth | Populations at carrying capacity |
| Hunted seals | 40 mm blubber | 25 mm blubber |
| By-caught seals | 35 mm blubber | 25 mm blubber |
Since all growing populations eventually approach the carrying capacity of the ecosystem unless they are controlled by hunting, predation or by stochastic events, vital population parameters will change. During recent years, the grey seal hunting quota substantially increased in both Finland and Sweden, which may affect the probability of reaching carrying capacity. In addition, resources in terms of available prey may fluctuate due to fishery activities. The seal abundance indicator within HELCOM has not declared that carrying capacity has been reached during the current assessment period.
3.1. Setting the threshold value(s)
Currently, this core indicator evaluates the nutritional status of only grey seals due to limited data and developmental stages of appropriate methodologies for harbour seals and ringed seals.
The threshold value for nutritional status is defined on what is considered to be a good condition in the current environment. To set the threshold value for grey seals, data on blubber thickness during the period 2001-2004 represents the most recent data period that indicated good status and is used to form a modern baseline for the threshold value concept for populations undergoing exponential growth. The threshold value is set at 40 mm blubber for samples from hunted seals and 35 mm blubber for by-caught seals. This threshold is currently applicable in the entire Baltic Sea since the population is highly migratory. Currently, data from 1 to 3 years-old grey seals of both sexes are used in this indicator. The blubber thickness of 1 to 3 years old grey seals shows a seasonal flux (HELCOM 2018). Due to this seasonality, seals collected from August to October are included in the evaluation.
4.1. Status evaluation
4.1.1. Grey seal
The current evaluation of the nutritional status of grey seals indicates that good status has not been achieved (cf. Figure 1). The status evaluation is based on 140 individuals (1 to 3 years old subadults) from Finnish and Swedish monitoring programmes, harvested in from August to October (cf. Table 3). The annual average blubber thickness does not reach the threshold throughout the entire assessment period. The distance to the threshold is larger for by-caught seals (8 mm below the threshold of 35 mm) and in some years (2018-2019) not even the proposed 25 mm threshold for carrying capacity is reached. However, it must be noted that the sample is very small so this result should be treated with caution.
Table 3. Average blubber thickness (mm) in hunted and by-caught grey seals (number of grey seals within brackets)
| Hunted, mm | Standard error, mm | Bycaught, mm | Standard error, mm | |
| 2016 | 37 (14) | 2.442 | 27 (8) | 1.563 |
| 2017 | 39 (6) | 5.231 | 32 (4) | 3.342 |
| 2018 | 32 (28) | 1.474 | 23 (3) | 3.055 |
| 2019 | 37 (35) | 1.205 | 24 (3) | 0.667 |
| 2020 | 38 (20) | 2.172 | – (0) | |
| 2021 | 34 (19) | 3.138 | – (0) | |
| Total | 36 (122) | 0.867 | 27 (18) | 1.277 |
4.1.2. Ringed seal
No evaluation was conducted due to lack of threshold and data constraints.
4.1.3. Harbour seal
No evaluation was conducted due to lack of threshold and data constraints.
4.2. Trends
Figure 2 shows the overall trend in yearly average blubber thickness from 2007 and onwards. The values are somewhat different from the results in the previous indicator report (HELCOM 2018) as three-year moving averages were not applied.
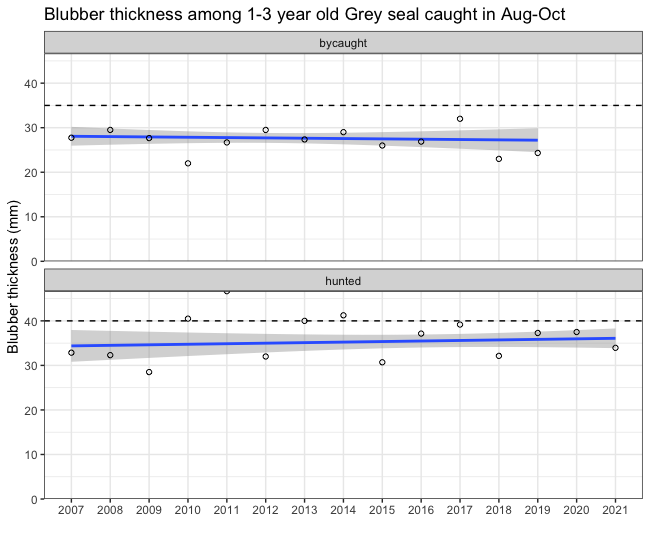
Figure 2. Blubber thickness (mm) for hunted and by-caught grey seals. All were by-caught or shot between August and December and between 1-3 years old. Dashed horizontal lines indicate the thresholds. The blue line is the linear regression trend line with a 95% point-wise confidence region shaded in grey.
4.3. Discussion text
The current evaluation is carried out on a limited dataset of grey seals but the inclusion criteria are well defined and therefore the variation in terms of seasonality, age-dependent changes and cause of death is minimised, which in turn increases the confidence in the evaluation. The yearly average blubber thickness of hunted seals during the assessment period varies between 32-39 mm (cf. Table 3) and does not seem to have decreased or increased during the last 15 years (Figure 2). The average blubber thickness is lower than during the reference period 2002-2004 which was used to set the threshold, so the good environmental status was not achieved.
Since the baseline was set, the average blubber thickness has declined (HELCOM 2018), but the Figure 2 for the last 15 years shows no alarming decreasing trend. However, there is a limit where the blubber becomes too thin for maintaining thermoregulatory effect. Analysis of fatty acid profiles in Baltic grey seal blubber revealed the presence of biochemically distinct vertical layers of outer, middle and inner layers (Tverin et al. 2019). The outer blubber layer is thermoregulative and significantly different from that of the thermoneutral middle and inner layers. In both grey seals and ringed seals, the outer blubber layer is about 15 mm thick. The middle layer, which expands and shrinks due to the nutritional status of the animal, has stable fatty acid composition, until about the last 9 mm of blubber adjacent to muscle, which show a fatty acid composition differing from the other blubber layers (Strandberg et al. 2008, 2011; Tverin et al. 2019). Also in harbour seals, a thermoregulatory outer blubber layer of 15 mm has been observed (Irwing & Hart, 1957). The separate layers presumably arise due to the dual role of blubber as a thermoregulatory and an energy storage tissue (Tverin et al. 2019). Seals with lower body weight and lower fat reserves show increased mortality (Kjellqwist et al. 1995, Harding et al. 2005, Bowen et al. 2015) and, in adults, decreased reproductive rate (Boyd et al. 1999). Blubber thickness also affects somatic growth and age at sexual maturity.
Further development of this indicator should aim to facilitate inclusion of more data. In the current evaluation, data had to be excluded due to lack of age determinations and the set inclusion criteria based on age, season and cause of death. The development should also be open to evaluations at smaller geographical scales. Local pressures will most likely not be reflected in the current indicator. For example, during 2020-2021 the grey seals in the southern Bothnian Bay suffered a significant decrease in blubber thickness. Juveniles had approximately 25 mm of blubber thickness in autumn during this time period. Investigations of health (i.e. necropsies) have not revealed a causative condition, although the frequency of colonic ulcers are generally high in adult seals from this area and increased during 2020-2021. At the same time, fishermen report depleted stocks of herring in this particular area. The herring and its fat content has previously been shown to be related to blubber thickness in grey seals (Kauhala et al. 2017). Diet analyses are pending and the investigations continue in 2022. Overall, the nutritional status indicator could be designed to better reflect local changes such as these. The example also serves to highlight the need for a broad investigation of possible causes behind an observed reduction in blubber thickness.
There is currently no literature to support different blubber thickness baselines in the north compared to the south of the Baltic Sea and it was not possible to investigate this in the reported data.
Sufficient material is collected annually for grey seals in Finland and Sweden to enable a status evaluation to be made, and the methodological approach is sound, thus the confidence of the indicator status evaluation for the grey seal in the central and northern parts of the Baltic Sea is high. Samples used in this evaluation also include Swedish material from the southern Baltic Sea and considering the known dispersive nature of the grey seal and the single population of grey seals in the Baltic Sea region, the scaling of the evaluation to the whole Baltic Sea is considered to have intermediate confidence. In the future, it would be highly beneficial to also include existing and future data from other countries (e.g. Denmark, Germany and Poland).
For harbour seals and ringed seal, although material is collected annually, further studies and analysis are required before status can confidently be evaluated.
Table 4. Brief summary of relevant pressures and activities with relevance to the indicator.
| | General | MSFD Annex III, Table 2a |
| Strong link | Hunting
By-catches. Disturbance causing stress. Ecosystem changes (food web, introduction of pathogens and non-indigenous species). Fishery and food availability. Fish quality |
Theme: Biological
|
7 Climate change and other factors
Climate change poses a pressure on species breeding on ice because shorter and warmer winters lead to more restricted areas of suitable ice fields (Meier et al. 2004). This feature alone will severely affect the Baltic ringed seals and the predicted rate of climate warming is likely to cause extirpation of the southern subpopulations (Sundqvist et al. 2012). Grey seals are facultative ice breeders and their breeding success is considerably greater when they breed on ice as compared on land (Jüssi et al. 2008). Furthermore, the weaning weight of grey seal pups was substantially greater when born on ice as compared with land. When a larger proportion of the grey seal pups are born on land in the future, they will likely be leaner and experience greater juvenile mortality. Consequently, both ringed seals and grey seals are predicted to be negatively affected by a warmer climate.
Temperature variations across the latitudinal extent of the Baltic Sea have been suggested to influence certain biological processes or community factors, however there is currently no documented evidence for a spatial variation in regulation of blubber thickness in subadult seals. It is likely that the average fat layer variations in grey seals between years represent changes in food availability and other stressors and not sea water temperature, i.e. possibly an indirect effect of a changing climate.
The grey seal nutritional status indicator does not achieve good environmental status during the years 2016-2021.
There is room for extending the indicator so that more data can be included, but this requires intensive work put into indicator development. Indicator development could be facilitated by research initiatives.
8.1. Future work or improvements needed
Criticism for the current indicator outline is the exclusion of large amount of data by not incorporating animals of additional age classes in addition to the current juveniles, for example age class 0 (pups of the year) and sexually mature females and males that could be included if care is taken to also account for their reproductive status. In addition, hunting is not conducted throughout the entire Baltic Sea and collection of seals found dead is the major source of data in those areas, so several countries can only contribute with data on live-caught, bycaught or even stranded animals. A wider sampling scope with stranded seals will increase the variation of the data (as they have as a group different causes of death), which in turn complicates the setting of a new threshold. There is currently no consensus on how to include stranded seals into the indicator. One possibility could be to have a separate threshold for stranded animals, as there are separate thresholds for bycaught and hunted animals. This topic of improvement requires further consideration.
Importantly, data collection and reporting through agreed national monitoring programs to a designated database need to be developed and expanded for all seal species in all relevant areas of their distribution aiming to to increase the spatial coverage of the data underlying the evaluation. Major methodological developments are also required to develop and agree on suitable thresholds for species of seals other than the grey seal. Aspects of this work will require new methodological approaches to the existing data and research initiatives. For the grey seal thresholds, spatial differences in blubber thickness and changes due to population dynamics should be investigated.
Initiatives to measure the body condition using drones are underway, which could result in a novel way of gathering large amounts of data. In addition, an initiative to collect data from hunters on all shot and retrieved seals in Sweden will hopefully result in reliable estimates on a more local scale. The drawback is that there is no additional necropsy data to explain any variations seen in blubber thickness.
9.1. Scale of assessment
This core indicator evaluates the nutritional status of seals using HELCOM assessment unit scale 2 (division of the Baltic Sea into 17 sub-basins). The assessment units are defined in the HELCOM Monitoring and Assessment Strategy Annex 4. Existing management plans for seals operate according to management units that are based on the distribution of seal populations. The management units typically encompass a handful of HELCOM scale 2 assessment units. Evaluations are therefore done by grouping HELCOM assessment units to align with the management units defined for each seal population. For the current indicator evaluation, grey seals spatial units in the Baltic Sea have been merged and are treated at the scale of the whole Baltic Sea (HELCOM scale 1), with the exclusion of the Kattegat and Limfjord unit.
- The Baltic grey seal (excluding Kattegat and the Limfjord) is a single management unit, although genetic data show some spatial structuring (Fietz et al. 2013). Data is available both from land-based surveys starting in the mid-1970s and later aerial surveys.
- The Baltic ringed seal is distributed in the Gulf of Bothnia (one unit – northerly) and Southwestern Archipelago Sea, Gulf of Finland and Gulf of Riga (second unit – more southerly), representing two different management units. This sub-division is justified by ecological data that indicate separate dynamics of the stocks (see HELCOM 2018).
- Harbour seals in the Kalmarsund, Sweden, constitute a separate management unit and is the genetically most divergent of all harbour seal populations in Europe (Goodman 1998). It was founded about 8,000 years ago and was close to extinction in the 1970s as a consequence of intensive hunting, and possibly also impaired reproduction (Härkönen et al. 2005) due to pollution. The genetic diversity is substantially reduced compared with other harbour seal populations.
- Harbour seals in the southwestern Baltic (Danish Straits, Danish, German and the Öresund region including Skåne county in Sweden and Kattegat) should be managed separately, as this stock is genetically distinct from adjacent populations of harbour seals (Olsen et al. 2014).
- Harbour seals in Kattegat and the Limfjord are genetically distinct from adjacent populations and each other (Olsen et al. 2014), but they are treated as one management unit.
9.2. Methodology applied
Currently, this core indicator evaluates the nutritional status of only grey seals due to limited data and developmental stages of appropriate methodologies for other species.
The current analysis is made using samples from sub-adult seals (1-3 years old) collected in August-October. The average blubber thickness measured at the sternum in millimetres. The average blubber thickness over the whole assessment period is evaluated against the set threshold (Table 1).
9.3. Monitoring and reporting requirements
HELCOM monitoring guidelines for nutritional status of seals were updated and accepted on EG HELCOM MAMA meeting in 2021.
The monitoring methodology is described in detail in the core indicator report from 2013 and 2018.
The monitoring activities relevant to the indicators that are currently carried out by HELCOM Contracting Parties are described in the HELCOM Monitoring Manual in the Monitoring Concept Table.
Current monitoring is carried out on a national basis, but initiatives of coordinating methodology have been taken by the Marine Mammal Health Team of the HELCOM Expert Group on Marine Mammals (EG MAMA).
Optimal monitoring should expand the current data collection to encompass the entire region in which the relevant seal species occur, and reporting through a dedicated data call and database should be developed.
The data and resulting data products (e.g. tables, figures and maps) available on the indicator web page can be used freely given that it is used appropriately and the source is cited.
HELCOM (2018) Nutritional status of marine mammals. HELCOM core indicator report. Online. [Date Viewed], [Web link].
Result: Nutritional status of seals
Data: Nutritional status of seals
The data collected and used in the indicator are based on national databases. The Marine Mammal Health Team of the HELCOM Expert Group on Marine Mammals is given the responsibility to compile, store current national data, and investigate future arrangements for establishing a HELCOM database.
This version of the HELCOM core indicator report was published in April 2023:
The current version of this indicator (including as a PDF) can be found on the HELCOM indicator web page.
Earlier versions of the indicator are available at:
Nutritional status of seals HELCOM core indicator 2018 (pdf)
Core indicator report – web-based version December 2015 (pdf)
Extended core indicator report – outcome of CORESET II project (pdf)
Bowen, W.D, den Heyer, C.E., McMillan, J.I., & Iverson, S.J. (2015) Offspring size at weaning affects survival to recruitment and reproductive performance of primiparous gray seals. Ecology and Evolution v.5(7): April 2015.
Boyd, I. L., Lockyer, C. & Marsh, H. D. (1999) Reproduction in marine mammals. In Biology of marine mammals (eds J. E. Reynolds & S. A. Rommel), pp. 218–286. Washington, DC: Smithsonian Institute Press.
European Commission (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora (Habitats Directive). Off. J. Eur. Union 206: 7–50.
European Commission (2008) Directive 2008/56/EC of the European Parliament and the Council establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union L 164: 19-40.
European Commission (2010) Commission Decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters (2010/477/EU). Off. J. Eur. Union L232: 12-24.
European Commission (2016) Draft Commission Decision laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU.
European Commission (2016) Draft Commission Directive amending Directive 2008/56/EC of the European Parliament and of the Council as regards the indicative lists of elements to be taken into account for the preparation of marine strategies.
Fietz, K., Graves, J.A., Olsen, M.T. (2013) Control Control Control: A Reassessment and Comparison of GenBank and Chromatogram mtDNA Sequence Variation in Baltic Grey Seals (Halichoerus grypus). PLoS ONE 8(8): e72853.
Fowler, C.W. (1981) Density dependence as related to life history strategy. Ecology 62: 602-610.
Goodman, S.J. (1998) Patterns of extensive genetic differentiation and variation among European harbor seals (Phoca vitulina vitulina) revealed using microsatellite DNA polymorphisms. Molecular Biology and Evolution 15(2): 104-118.
HELCOM (2018) Nutritional status of seals. HELCOM Core Indicator Report. Online. <http://www.helcom.fi/Core%20Indicators/Nutritional%20status%20of%20seals%20HELCOM%20core%20indicator%202018.pdf>.
Haller, M.A., Kovacs, K.M., Hammill, M.O. (1996) Maternal behaviour and energy investment by grey seals (Halichoerus grypus) breeding on land fast ice. Can. J. Zool. 74: 1531-1541.
Harding, K.C., Fujiwara, M., Härkönen, T., Axberg, Y. (2005) Mass dependent energetics and survival in harbour seal pups. Functional Ecology 19: 129-135.
Harwood, J., Prime, J.H. (1978) Some factors affecting the size of British grey seal populations. Journal of Applied Ecology: 401-411.
Hauksson E., Bogason, V. (1997) Comparative feeding of grey (Halichoerus grypus) and common seals (Phoca vitulina) in coastal waters off Iceland, with a note on the diet of hooded (Cystophora cristata) and harp seals (Phoca groenlandica). J. Northwest Atl. Fish. Sci. 22: 125–135.
Härkönen, T., Harding, K.C. (2001) Spatial structure of harbour seal populations and the implications thereof. Can. J. Zool. 79: 2115-2127.
Härkönen, T., Harding, K.C., Goodman, S., Johannesson, K. (2005) Colonization history of the Baltic harbor seals: Integrating archaeological, behavioural and genetic data. Marine Mammal Science 21: 695-716.
Irving L, Hart JS (1957) The metabolism and insulation of seals as
bare-skinned mammals in cold water. Can J Zool 35:497–511
Jüssi, M., Härkönen, T., Jüssi, I., Helle, E. (2008) Decreasing ice coverage will reduce the reproductive success of Baltic grey seal (Halichoerus grypus) females. Ambio 37: 80–85.
Kauhala, K., Bäcklin, B. M., Raitaniemi, J., & Harding, K. C. (2017). The effect of prey quality and ice conditions on the nutritional status of Baltic gray seals of different age groups. Mammal research, 62(4), 351-362.
Kjellqwist, S.A., Haug, T., Øritsland, T. (1995) Trends in age composition, growth and reproductive parameters of Barents Sea harp seals (Phoca groenlandica). ICES J. Mar. Sci. 52(2): 197–208.Kovacs, K.M., Lavigne, D.M. (1986) Growth of grey seal (Halichoerus grypus) neonates: different maternal investment in the sexes. Can. J. Zool. 64: 1937-1943.
Krafft, B.A., Kovacs, K.M., Frie, A.K., Haug, T., Lydersen, C. (2006) Growth and population parameters of ringed seals (Pusa hispida) from Svalbard, Norway, 2002-2004. ICES Journal of Marine Science 63: 1136-1144.
McCann, T.S., Fedak, M.A., Harwood, J. (1989) Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology. 25: 81-87
Meier, H.E.M., Döscher, R., Halkka, A. (2004) Simulated distributions of Baltic Sea-ice in the warming climate and consequences for the winter habitat of the Baltic Ringed Seal. Ambio 33: 249–256.
Nilssen, K. T., Haug, T., Lindblom, C. (2001) Diet of weaned pups and seasonal variations in body condition of juvenile Barents Sea harp seals Phoca groenlandica. Marine Mammal Science 17: 926–936.
Olsen, M.T., Wesley Andersen, L., Dietz, R., Teilmann, J., Harkonen, T., Siegismund, H.R. (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology 23: 815-831.
Strandberg U., Käkelä A., Lydersen C., Kovacs K.M., Grahl-Nielsen O., Hyvärinen H., Käkelä R. (2008) Stratification, composition, and unction of marine mammal blubber: the ecology of fatty acids in marine mammals. Physiol Biochem Zool 81:473–485
Strandberg U, Sipilä T, Koskela J, Kunnasranta M, Käkelä R (2011) Vertical fatty acid profiles in blubber of a freshwater ringed seal— comparison to a marine relative. J Exp Mar Bio Ecol 407:256–265
Sundqvist, L., Harkonen, T., Svensson, C.J., Harding, K.C. (2012) Linking climate trends to population dynamics in the Baltic ringed seal – Impacts of historical and future winter temperatures. Ambio.
Tverin M, Westberg M, Kokkonen I, Tang P, Lehmann P, Lundström K, Käkelä R. (2019) Factors affecting the degree of vertical stratification of fatty acids in grey seal blubber. Marine Biology 166:105.
Bäcklin, B.-M., Moraeus, C., Roos, A., Eklöf, E., Lind, Y. (2011) Health and age and sex distributions of Baltic grey seals (Halichoerus grypus) collected from bycatch and hunt in the Gulf of Bothnia. ICES Journal of Marine Science 68: 183-188.
Bergman, A., Olsson, M. (1985) Pathology of Baltic grey seal and ringed seal females with special reference to adrenocortical hyperplasia: Is environmental pollution the cause of a widely distributed disease syndrome. Finnish Game Res 44: 47-62.
Fedak, M., Anderson, S. (1987) The energetics of sexual success of grey seals and comparison with the costs of reproduction in other pinnipeds. Symp. Zool. Soc. Lond. 57: 319-341.
Harkonen, T., Bäcklin, B.-M., Barrett, T., Bergman, A., Corteyn, M., Dietz, R., Harding, K., Malmsten, J., Roos, A., Teilmann, T. (2008) Mass mortality in harbour seals and harbour porpoises caused by an unknown pathogen. The Veterinary Record 162: 555-556.
Harkonen, T., Jüssi, M., Jüssi, I., Verevkin, M., Dmitrieva, L., Helle, E., Sagitov, R., Harding, K.C. (2008) Seasonal activity budget of adult Baltic ringed seals (Phoca hispida botnica). PLoS ONE 3(4): e2006.doi:10.1371/journal.pone.0002006.
Harkonen, T., Isakson, E. (2011) Historical and current status of harbour seals in the Baltic proper. NAMMCO Scientific Publications 8: 71-76.
Kauhala, K., Kurkilahti, M., Ahola, M.P., Herrero, A., Karlsson, O., Kunnasranta, M., Tiilikainen, R., Vetemaa, M. (2015) Age, sex and body condition of Baltic grey seals: Are problem seals a random sample of the population? Annales Zoologici Fennici52: 103–114.
Oksanen, S. M., Niemi, M., Ahola, M. P. & Kunnasranta, M. 2015. Identifying foraging habitats of Baltic ringed seals using movement data. Movement Ecology 3: 33 (1–11). DOI 10.1186/s40462-015-0058-1
Olsen, M.T., Wesley Andersen, L., Dietz, R., Teilmann, J., Harkonen, T., Siegismund, H.R. (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology 23: 815-831.
Ryg, M., Lydersen, C., Markussen, N. H., Smith, T. G., Øritsland, N. A. (1990) Estimating the blubber content of phocid seals. Canadian Journal of Fisheries and Aquatic Sciences 47(6): 1223-1227.
Stephens, P.A., Houston, A. I., Harding, K.C., Boyd, L., McNamara, J.M. (2014) Capital and income breeding in pinnipeds: the role of food supply. Ecology. http://www.esajournals.org/doi/abs/10.1890/13-1434.1
Vanhatalo, J., Vetemaa, M., Herrero, A., Aho, T., Tiilikainen, R. (2014) By-Catch of Grey Seals (Halichoerus grypus) in Baltic Fisheries—A Bayesian Analysis of Interview Survey. PLoS ONE 9(11): e113836. doi:10.1371/journal.pone.0113836.
Bergman, A. (1999) Health condition of the Baltic grey seal (Halichoerus grypus) during two decades. Apmis 107(1‐6): 270-282.
Dietz, R., Heide-Jørgensen, M.-P., Härkönen, T. (1989) Mass deaths of harbour seals Phoca vitulina in Europe. Ambio 18(5): 258-264.
Harding, K.C., Härkönen, T., Caswell, H. (2002) The 2002 European seal plague: epidemiology and population consequences. Ecology Letters 5: 727-732.
Harding, K.C., Härkönen, T., Helander, B., Karlsson, O. (2007) Status of Baltic grey seals: Population assessment and risk analysis. NAMMCO Scientific Publications 6: 33-56
Härkönen, T., Stenman, O., Jüssi, M., Jüssi, I., Sagitov, R., Verevkin, M. (1998) Population size and distribution of the Baltic ringed seal (Phoca hispida botnica). In: Ringed Seals (Phoca hispida) in the North Atlantic. Edited by C. Lydersen and M.P. Heide-Jørgensen. NAMMCO Scientific Publications 1: 167-180.
Härkönen, T., Dietz, R., Reijnders, P., Teilmann, J. Harding, K., Hall, A., Brasseur, S., Siebert, U., Goodman, S., Jepson, P., Dau Rasmussen, T., Thompson, P. (2006) A review of the 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Diseases of Aquatic Organisms 68: 115-130.
Härkönen, T., Brasseur, S., Teilmann, J., Vincent, C., Dietz, R., Reijnders, P., Abt, K. (2007) Status of grey seals along mainland Europe, from the Baltic to France. NAMMCO Scientific Publications 6: 57-68.
Härkönen, T., Harding, K., Rasmussen, T.D., Teilmann, J., Dietz, R. (2007) Age- and Sex-specific Mortality Patterns in an Emerging Wildlife Epidemic: the Phocine Distemper in European Harbour Seals. PLoS ONE 2(9): e887. doi: 10.1371/journal.pone.0000887.
Olsen, M.T., Andersen, S.M., Teilmann, J., Dietz, R., Harkonen, T. (2011) Status of the harbour seal in Southern Scandinavia. NAMMCO Scientific Publications 8: 77-94.
Svensson, C.J., Hansson, A., Harkonen, T., Harding, K. (2011) Detecting density dependence in growing seal populations. Ambio (2011) 40: 52–59.