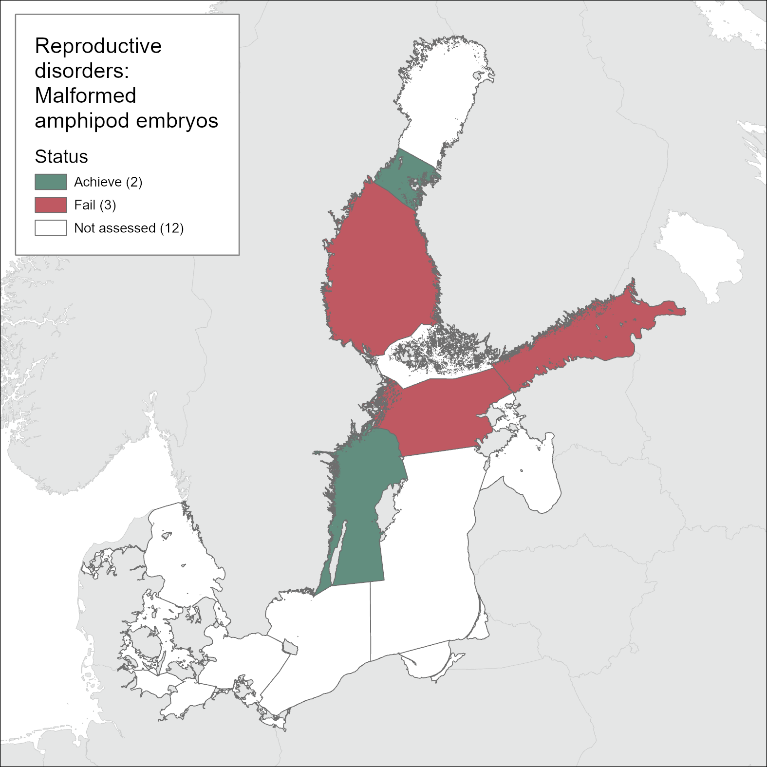
Reproductive disorders
Reproductive disorders
2 Relevance of the indicator
2.1 Ecological relevance
The elevated frequencies of malformed embryos are regarded as a significant biological response for assessing the population-relevant effects induced by the combined exposure to the environmental contaminants in the sea sediments. The indicator provides information on reproductive success and thereby population health, persistence and stability.
In the Baltic Sea, malformed embryos in Monoporeia affinis and Pontoporeia femorata have been used as a bioindicator for reproductive toxicity caused by pollutants for the last 20 years. These are the keystone species in the Baltic Sea and freshwater ecosystems below the highest coastline. M affinis is one of the most abundant macrofauna species in soft bottoms (10 to 150 m), provided that oxygen conditions are sufficient (Kuparinen et al. 1996). Amphipods are very important for the oxygenation of the sediment by bioturbation (Lindström 1992), they are also food for fish, such as herring, eelpout, cod and flounder, as well as other invertebrates i.e. Saduria entomon, Halicryptus spinulosus and Bylgides sarsi (Ankar and Sigvaldsdottir 1981, Aneer 1975, Sparrevik and Leonardsson 1995). The Baltic M. affinis populations have decreased dramatically during the last 30 years, and currently the species abundances in the Gulf of Finland and Gulf of Gdansk are very low. The population crash in the year 2000 resulted in dramatically decreased populations in the Gulf of Bothnia (Eriksson Wiklund et al. 2008).
Other amphipods used in the monitoring (P. robustoides, G. tigrinus, G. fasciatus) belong to so-called alien species, but they are also important components (30-40% of the total biomass) in the benthic communities in coastal areas of the Baltic Sea (Gulf of Riga, Gulf of Finland, Curonian and Vistula Lagoons) since 1990s and are the main prey for local fish and birds. These species are omnivores, with more than 50 % of detritus in their diet (Berezina 2007). These gammarids are widely used as test indicators for sediment toxicity (Berezina et al. 2017; Strode et al. 2017). All of them have a life span of 1.5 year; mating begins in April-May, embryogenesis takes 2-3 weeks, and juveniles of the 2-3 generations are released during summer (Panov and Berezina 2002; Bacela and Konopacka, 2005, Berezina et al. 2011).
Reproductive disorders in amphipods
Amphipod embryos are sensitive to sediment toxicity during the embryogenesis and various embryo aberrations can occur in response to toxic exposure (Figure 2). Moreover, the frequency of malformed embryos is more sensitive to contaminant exposure than other reproduction variables, such as fecundity, sexual maturation, and fertilization rate (Sundelin, 1983; Sundelin and Eriksson, 1998). Many chemical pollutants induce various embryo aberrations, which makes them useful as ecologically relevant indicators for chronic biological effects of environmental contaminants. For example, in amphipods, PAHs and PCBs increase the frequency of malformed and membrane-damaged embryos (Löf et al. 2016), and the frequency of embryos with arrested development increases due to elevated concentrations of some PAHs and metals (Löf et al. 2016). In bioassays, exposure of M. affinis to metals (e.g., As, Cd, Pb) and sediments collected nearby pulp mill discharges caused higher frequencies of malformed and membrane-damaged embryos compared to reference sediments (Sundelin, 1983, 1984, 1989; Blanck et al., 1989; Wiklund et al., 2005). Genotoxicity and epigenetic changes have been linked to embryo aberrations in the Baltic M. affinis (Gorokhova e al. 2021), indicating that changes might be persistent in the populations and affect their stability.
All amphipod species have similar embryo development and the method proposed here is applicable to all species. Embryo aberrations (see Table 2 in Löf et al. 2016) are classified as: (1) malformed embryo, with aberrant morphology of extremities and body symmetry, (2) embryo with a damaged membrane; (3) embryo with arrested development (AD) and (4) dead or partially dead broods. Categories 1 to 3 were found to be most representative of contaminant-induced developmental toxicity and these are the categories used under the collective name malformed embryos in the indicator evaluation.
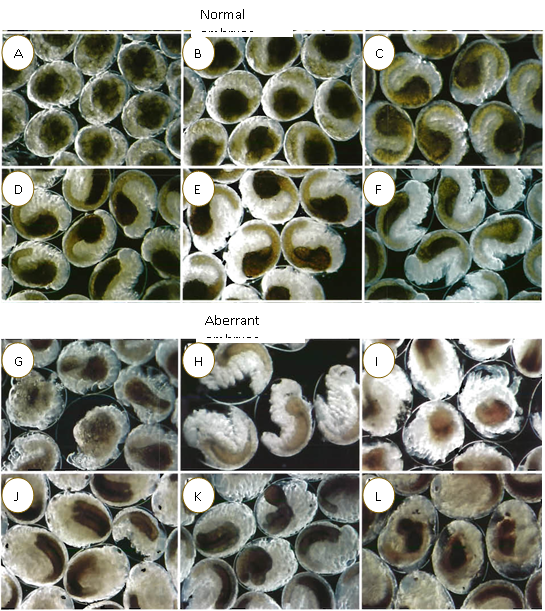
Figure 2. Normal (A-F) and aberrant (G-L) embryos of Monoporeia affinis of different developmental stages (modified from Sundelin and Eriksson, 1998. When embryo analysis is conducted (January-February), these are the most common stages present and dispaying various developmental pathologies.
Normal embryos: A, B: Stage 5; C, D: Stage 6, E, F; Stage 7;
Aberrant embryos: G, H: Oedema and impaired membranes with lipids leaking between the inner and the outer egg membranes; I, J: Embryos with irregular midgut, shortened limbs or irregular comma-like compound eye; K, L: Non-specific multiple malformations of limbs and gut
Various amphipods and some fish species can be used for biological effects indicator-based evaluations. The approach was initially developed for Monoporeia affinis, the keystone species in the Baltic Sea. Other amphipod species, such as Gmelinoides fasciatus, Pontogammarus robustoides and Gammarus tigrinus, that were used for the indicator development in the eastern Gulf of Finland are key members in the benthic communities in the coastal areas of the Baltic Sea (Gulf of Riga, Gulf of Finland, Curonian and Vistula Lagoons) and an important prey of local fish and birds. Various gammarids, i.e., G. salinus, G. zaddachi, and G. tigrinus, are common in coastal littoral communities and can also be used for ReproIND-based evaluation (Reproductive disorders based evaluation).
2.2 Policy relevance
The marine environment, especially sediment, is the ultimate repository for complex mixtures of persistent chemicals. Extreme exposure (e.g. high levels or sudden events) can result in direct toxicity and death, but long-term chronic exposure is also highly significant. Baltic Sea organisms are exposed to a range of substances, many of which can cause metabolic disorders and, may affect populations through changes in growth, reproduction, and survival. Evaluation of the biological perturbations as a consequence of exposure to hazardous substances is a prerequisite for environmental quality assessment. As a biological effect indicator, the reproductive disorders are important for the evaluation of the integrated effects of the bioavailable contaminants with strong repercussion at the population level. This goal is included in the Baltic Sea Action Plan (BSAP 2021) and the Marine Strategy Framework Directive (MSFD), as set out in Table 1 below. Under the BSAP (2021) this topic is also the focus of Action HL13: ‘By 2028 develop further relevant monitoring for the biological effects of hazardous substances in order to facilitate a reliable ecosystem health assessment’. Whereas many effect variables and biomarkers (indicators) are not specific and respond to various environmental stressors, both natural and anthropogenic, ‘Reproductive disorders’ is an indicator that is comparatively specific and responds mainly to the contaminant exposure (Löf et al. 2016, Gorokhova et al. 2020).
Table 1. Policy relevance of the indicator.
| Baltic Sea Action Plan (BSAP) | Marine Strategy Framework Directive (MSFD) | |
| Fundamental link | Segment: Hazardous substances and litter goal
Goal: “Baltic Sea unaffected by hazardous substances and litter”
|
Descriptor 8 Concentrations of contaminants are at levels not giving rise to pollution effects.
|
| Complementary link | Descriptor 9 Contaminants in fish and other seafood for human consumption do not exceed levels established by Union legislation or other relevant standards.
(a) for contaminants listed in Regulation (EC) No 1881/2006, the maximum levels laid down in that Regulation, which are the threshold values for the purposes of this Decision; (b) for additional contaminants, not listed in Regulation (EC) No 1881/2006, threshold values, which Member States shall establish through
|
|
| Other relevant legislation: |
|
|
2.3 Relevance for other assessments
The status of hazardous substances is currently assessed using several core indicators, almost exclusively contaminant concentrations. Thus, each indicator focuses on one important aspect of the contaminant distribution, levels, and possible effects. However, given the recent shift from high concentrations of a relatively small number of chemicals to low concentrations of many, there is a need for effect-based methods to assess in situ toxicity of chemical mixtures and to facilitate the interpretation of the concentration-based indicators (ICES 2021). Currently, this biological effect indicator is not integrated into the overall assessment of contamination status; however, it provides supplementary information and should be considered together with other hazardous substances core indicators in order to evaluate the overall status of hazardous substances in the Baltic Sea.
The current integrated assessment of hazardous substances utilises mainly substance concentrations and this is the focal point of the HOLAS Thematic Assessment on Hazardous Substances/Pollution. This indicator is however included within the contextual discussion and there is an aim to develop test cases for an Integrated Biological Effects of Contaminants (I-BEC) pilot study in HOLAS 3 to which relevant results from this indicator will contribute.
3 Threshold values
3.1 Setting the threshold values
The embryo malformation indicator for amphipods is a multimetric indicator based on two variables measured in the sampled population, namely
(1) the proportion of malformed embryos and
(2) the proportion of females with more than one malformed embryo.
These two variables are measured using the same pool of field-collected gravid females; to achieve good status for an area, both variables must be below or equal to their respective threshold values (Figure 3).
The threshold value is established based on the monitoring data using a target-setting approach with percentiles of the normal distribution as the threshold value as recommended by ICES for biological effect indicators (Davies and Vethaak 2012). The not-good status (i.e. failure to achieve the threshold value) is defined as a significant increase in embryo malformation frequency or a significant increase in the frequency of females carrying more than one malformed embryo compared to the background level. The threshold values do not change over time, and the same approach is proposed to be applied throughout the Baltic Sea in all assessment units.
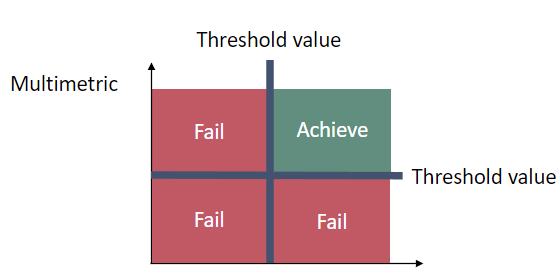
Figure 3. Schematic representation of the threshold value indicates good status when both the proportion of malformed embryos and the proportion of females with more than one malformed embryo are below their respective threshold values.
For the amphipod Monoporeia affinis, data from the Swedish National Marine Monitoring Program (SNMMP) were used to establish the workflow, determine the baselines (as mean percentage in the population) and the thresholds for the percentage of malformed embryos and the percentage of females with more than one malformed embryo (Table 2 and figure 4). The data used to derive the thresholds (Figure 4) are based on gravid females collected at fourteen stations from 1994 to 2011. Background assessment criteria (BAC) have been derived from the median values of the 90th percentiles for the data selected from the areas that were regarded as relatively unpolluted reference areas in the Baltic Sea, with no known point sources. The lower bound of environmental assessment criteria (EAC) was set at the 90th percentile value for each variable (Davies and Vethaak 2012). Bootstrapping (100 000 runs) was used to derive mean, median, and 90th percentile values for the embryo aberration frequency and frequency of the females with more than 1 malformed embryo in the population. All types of embryo malformations (embryos with malformed limbs, eyes, and midgut, membrane-damaged embryos, and embryos with arrested development) were included in the category malformed embryos because all these developmental aberrations are lethal and all of them were found to be associated with exposure to various contaminants (Löf et al. 2016).
Table 2. Threshold values for the amphipod Monoporeia affinis. Background assessment criteria (BAC) and environmental assessment criteria (EAC) are adopted from Davies and Vethaak (2012). BoS: Bothnian Sea, NBP: Northern Baltic Proper, WGB: Western Gotland Basin, GoF: Gulf of Finland and GoR: Gulf of Riga. As no difference in the baseline values for embryo malformation rate was detected between the basins in the Swedish waters for which the monitoring data are available, the same threshols are proposed for Quark, BoS, NBP and WGB.
| Subbasin | Assessment criteria | Mean | BAC | EAC |
| Quark, BoS, NBP and WGB | Proportion of aberrant embryos | 0.041 | 0.06 | >0.06 |
| Proportion of females with >1 aberrant embryo | 0.23 | 0.30 | >0.30 | |
| GoF | Proportion of aberrant embryos | 0.05 | 0.07 | >0.07 |
| Proportion of females with >1 aberrant embryo | 0.40 | 0.52 | >0.52 | |
| GoR | Proportion of aberrant embryos | 0.04 | 0.06 | >0.06 |
| Proportion of females with >1 aberrant embryo | 0.14 | 0.22 | >0.22 |
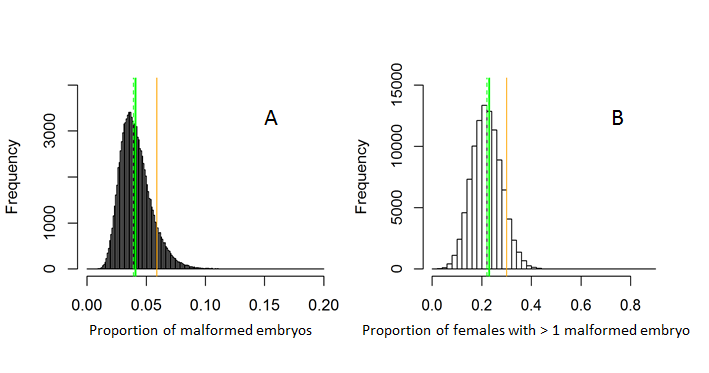
Figure 4. Defining thresholds for (A) Proportion of malformed embryos and (B) Proportion of females with > 1 malformed embryo in the population of the amphipod Monoporeia affinis in the Gulf of Bothnia, Western Gotland Basin, and Northern Baltic Proper. The solid green line indicates the background level (mean value in the population), the dotted green line denotes the median, and the orange line denotes the threshold value (90th percentile), beyond which the malformation rate is significantly higher than the background levels.
In areas where M. affinis does not occur naturally or is found sporadically and/or at low abundances, other amphipods with a similar life cycle and reproduction biology can be used to derive the embryo malformation indicator. For example, in the Gulf of Finland and the Gdansk Bay, where M. affinis is relatively rare, secondary thresholds were established for other amphipod species belonging to gammarids (Table 3). As with M. affinis, these thresholds involve two values: one for the percentage of malformed embryos and another for the percentage of females carrying more than one malformed embryo.
Table 3. Secondary thresholds for the amphipods Gmelinoides fasciatus, Pontogammarus robustoides and Gammarus tigrinus (Gammarida). The values are based on Gulf of Finland monitoring data (Russia).
| Subbasin | Assessment criteria | Mean | BAC | EAC |
| GoF | Proportion of aberrant embryos | 0.02 | 0.05 | >0.05 |
| Proportion of females with >1 aberrant embryo | 0.15 | 0.20 | >0.20 |
3.2 Sources of uncertainties in the threshold values
In various Baltic Sea areas, the baseline embryo malformation levels in Monoporeia affinis were found to be similar to those found for the monitoring sites, implying that the indicator is not sensitive to variations in salinity, depth, and food availability. At the same time, populations inhabiting areas with elevated concentrations of contaminants in the sediments or living close to known point sources showed a higher malformation rate, implying that the indicator is responsive to xenobiotics and represents a relevant measure of the biological effects of environmental contaminants.
There are certain assumptions in the target-setting approach when the latter is based on the percentiles of the normal distribution. One such assumption is that reference areas used to analyse the statistical distribution of the malformation rate are not negatively affected and represent pristine areas. However, in the Baltic Sea, such pristine areas are difficult to find. Due to the lack of unquestionably pristine reference sites, some uncertainty in the accuracy of the threshold value remains but is not deemed to be very substantial, due to a large amount of data available for target setting and broad coverage of the area (i.e., large number of stations) within each basin.
3.3 Data aggregation
For the basins with high spatial coverage of the sampling stations (the Bothnian Sea and Western Gotland Basin), the stations were grouped into regions, whereas for other basins (The Quark, Northern Baltic Proper, and the Gulf of Finland), no such grouping was applied. For the evaluation of different basins on the annual basis, a 50% rule has been applied which means that:
- Each station per year was evaluated individually;
- When stations grouped by regions were evaluated, the region was considered as being in good status when >50% of the stations within the region were in good status;
- All regions within a basin were evaluated, and the basin was considered as being in good status when >50% of the regions within the basin were in good status. If the evaluation was conducted using stations without the region grouping, the same principle was applied for the stations, i.e., the basin was considered as being in good status when >50% of the stations were in good status;
- The whole six-year assessment period was considered good if >3 years were in good status.
4 Results and discussion
The results of the indicator evaluation that underly the key message map and information are provided below. The spatial coverage of this indicator has increased for HOLAS 3 as the required parameters have been included in the HOLAS 3 data call and more Contracting Parties have provided data, resulting in 148 station/year observations available for the evaluation in 2016-2021 (Table 4).
The data synthesis was carried out by Estonia, Latvia, Russia, and Sweden using four amphipod species: Monoporeia affinis, Gammarus salinus, G. zaddachi, and G. tigrinus, whereas in HOLAS II the evaluation was based on M. affinis only. Based on the data synthesis, the new threshold values were derived for the M. affinis populations in the Gulf of Finland and the Gulf of Riga (Table 2) and used for the evaluation in the Gulf of Finland and analysis of the available data in the Gulf of Riga.
Table 4. Overview of the datasets used for ReproIND evaluation for the period 2016-2021. The sampling stations are referred to by their names used in ICES/DOME database, the national station registers and HELCOM map and data service. The number of females per year that were examined is shown as a mean and range (in parenthesis).
| Sub-basin | Countries providing data | Years evaluated | Number of females/year | Stations |
| The Quark | Sweden | 2016, 2020-2021 | 99 (86-110) | N 25, N 26, N 27 |
| Bothnian Sea | Sweden | 2016-2021 | 418 (346-510) | G 10, G 11, N 4-1, N 4-3, Åv 1, Åv 2, Su 4, Su 5, R 2-4, R 2-13, N 2-4, N 2-3, SR 1A |
| Northern Baltic Proper | Sweden | 2016-2020 | 150 (130-176) | KUD 64, SV 3, KO 2, SB 8, 1003 |
| Western Gotland Basin | Sweden | 2016-2020 | 241 (151-356) | 6020, 6025, 6004, Grund Utsjö, 6022, 6019, HAE 3,HAE13, HAE4, STANNA 6, STANNA 2, Gryt 1, Gryt 3 |
| Gulf of Finland | Estonia, Russia | 2016, 2020-2021 | 203 (151-241) | 2, 3, 18a, HR50, M, J3, N12, N, N8, U, KS, L, P, ER, 2F, 3F, 4F, 9F, 2ugms |
| Gulf of Riga | Latvia, Estonia | 2020-2021 | 157 (37-256) | 107, 111, 167B, 163B, 162B, 160B, 101A, 114A, VAD2, 111E, L9 |
4.1 Status evaluation
ReproIND has been applied in the two sub-basins shared by Finland and Sweden (the Quark and the Bothnian Sea), the Northern Baltic proper and Western Gotland Basin, and the Gulf of Finland (Figure 5). In the Gulf of Riga, the annual results were available only for 2020 and 2021. Consequently, it was not possible to assess the status in this basin, because at least three years of observations are needed to conduct the evaluation. The data available are, however, shown for comparative purposes (Figure 5f).
The evaluation for the assessment period 2016-2021 concluded that the Quark and the Western Gotland Basin have achieved good environmental status (GES), whereas the Bothnian Sea, Northern Baltic Proper, and the Gulf of Finland were not in good status. Deviations in frequencies of embryo malformations and of females carrying malformed embryos were apparent in all assessment units, except the Quark (Figure 5). Thus, the results indicate high reproductive and developmental toxicity in the amphipod populations inhabiting most of the evaluated areas.
The highest levels of reproductive aberrations in M. affinis, with the frequency of females with aberrant embryos >0.4 (reaching 1 in some cases) and frequency of malformed embryos >0.06 (reaching 0.14-0.19 in some cases), observed in the evaluated units during 2016-2021, are sufficient to cause high fluctuations in the amphipod stocks, adversely affect abundances, with increased probability of population extinction, as suggested by M. affinis population modelling study (Reutgard 2015). Although it is unlikely that the entire Baltic population can go extinct, the decreases in the local population abundance observed during the last years (Raymond et al. 2021) may become detrimental to the productivity of benthic communities, alter benthic-pelagic coupling and influence energy fluxes in soft-bottom sediments.
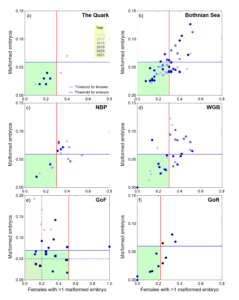
Figure 5. Assessment results on the performance of ReproIND (malformed embryos of amphipods), which integrates the frequency of malformed embryos (Y-axis) and frequency of females carrying more than one malformed embryo (X-axis) in the populations of Monoporeia affinis (circles) and gammarids (squares) in 2016-2021. Blue and red lines (solid lines: M. affinis and dashed lines: gammarids) show threshold values for the frequency of malformed embryos and the frequency of females carrying more than one malformed embryo, respectively. The green-shaded quartiles indicate good status based on the thresholds for M. affinis (solid color) and gammarids (diagonal pattern). Thus, observations in good status are those located in the green shaded area, and those not in good status are outside of it. Each data point represents a sampling station and a year: The Quark (1 region, 4 stations), the Bothnian Sea (BS; 4 regions, 13 stations), the Northern Baltic Proper (NBP; 1 region, 5 stations), the Western Gotland Basin (WGB; 3 regions, 13 stations), the Gulf of Finland (GoF; 1 region, 19 stations), and the Gulf of Riga (GoR; 1 region, 11 stations; Table 4); see Methodology section.
4.2 Trends
Even though GES in the Western Gotland Basin was achieved for the entire assessment period, the aberration frequencies were highly variable between the stations (data not shown) and between the years for the data aggregated by basin (Figure 6), indicating instability of the population status and toxicity of the sediments in the area. Moreover, both ReproIND components (e.g., frequency of the aberrant embryos and frequency of females with more than one aberrant embryo) have increased compared to HOLAS II (Figure 6). Notably, if the station-specific data were not aggregated by region, the evaluation would yield sub-GES as the outcome in HOLAS 3.
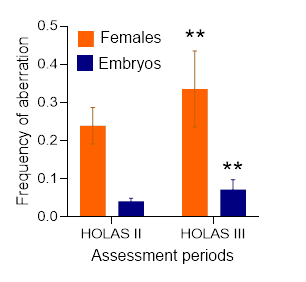
Figure 6. Comparison of the ReproIND components (frequency of the aberrant embryos and frequency of females; mean values and standard deviations for each parameter) for M. affinis in the Western Gotland Basin between the assessment periods. The data from all stations within the basin (see Figure 8) were aggregated and analysed by the 2-way ANOVA, which confirmed significantly higher ReproIND values in HOLAS 3 compared to HOLAS II (p < 0.0023).
To detect trends in ReproIND, long-term data is needed and collected and analysed in the same manner. Currently, such data are only available for the Swedish data series. Analysis of the trends for these data indicates that a significant decline of the aberration frequencies in the Northern Baltic Proper and Western Gotland Basin has occured during the last 15 years (Kendall test, p < 0.05).
4.3 Discussion text
Status evaluations, though useful and informative, do not provide the full details that may be critical for effective management. This can be evidenced from the comparison between assessment periods made below (Figure 7 and Table 5) and highlights the need for information on trends, as outlined above. By directly comparing only the status evaluation between HOLAS II and HOLAS 3 periods there is no change identified between the two periods (Figure 7). However, as described above without also exploring trends across time series as well as comparisons between assessment periods it is not possible to identify key issues such as improvements or deteriorations in the evaluated parameter(s). There are a number of reasons, as well as the scientific value, for such analyses and these include, amongst others: deterioration may for example highlight a new or increased pressure, deterioration may inform of a failure in part of the management cycle, and improvement may provide the opposite information or indicate recovery in the ecosystem. Such changes that are potentially masked by focussing only on the final status evaluation and are vital for management action as they can inform for example on the need for measures, the targeting of actions, act as early warning systems or even inform on the success and sufficiency of measures/actions taken. A summary of relevant information is provided in Table 5.
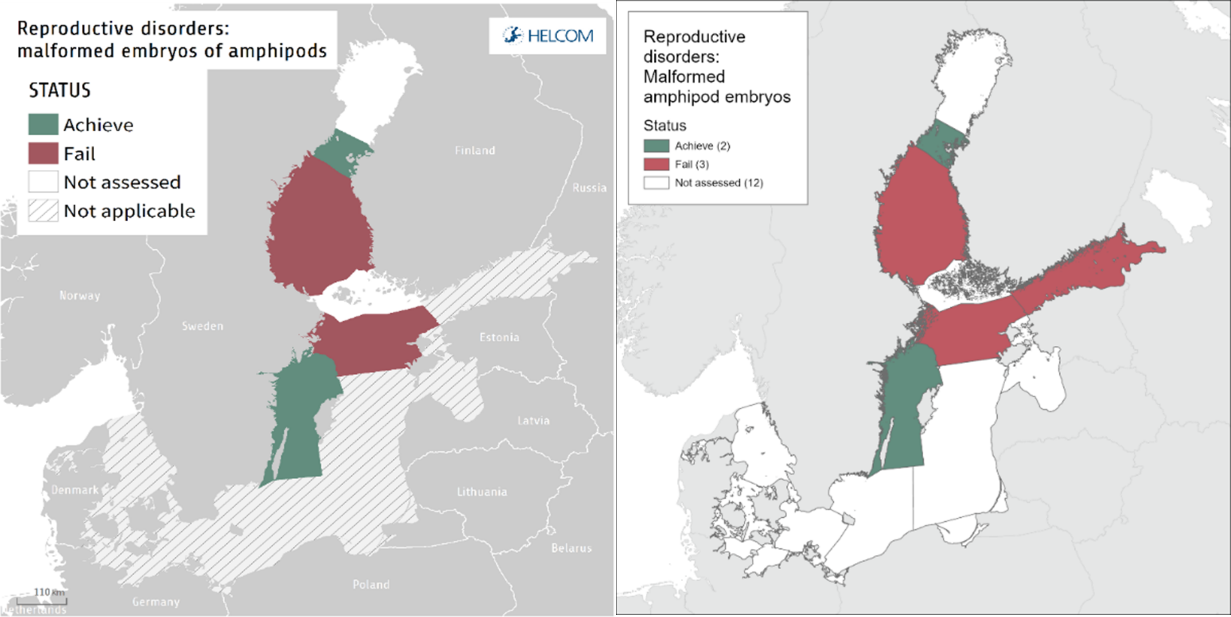
Figure 7. Status evaluation results for HOLAS II (left panel) and HOLAS 3 (right panel) are based on the evaluation of the indicator Reproductive disorders: malformed embryos of amphipods. The evaluation is carried out using scale 2 HELCOM assessment units (defined in the HELCOM Monitoring and Assessment Strategy Annex 4), combining coastal and offshore amphipod species and using species-specific target values. Not applicable is used for areas in which no agreement on the application of this indicator have currently been made. See ‘data chapter’ for interactive maps and data at the HELCOM Map and Data Service.
Table 5. Overview of the evaluation and comparison with earlier evaluations.
5 Confidence
The confidence of the indicator is high in the Bothnian Sea and Western Gotland Basin since more than 20 years of data following the same methodology for embryo analysis were used for setting the threshold values. These are also the areas where the highest number of gravid females collected from multiple stations and aggregated to regions surveyed on annual basis was used for the evaluation (Table 4). The confidence of the indicator is moderate in the Quark and the Northern Baltic Proper, because the spatial coverage and number of stations were somewhat lower (Table 4, Figure 5), although, considering a smaller total area of the Quark, this sampling coverage is comparable to that in the Bothnian Sea. The confidence of the indicator in the Gulf of Finland is also moderate because the number of females examined and the station coverage are sufficiently high, but the number of evaluation years is relatively low.
Whereas more than 20 years of data for setting the threshold values were used for the Swedish data, the time series for the Latvian and Estonian collections are much shorter. Therefore, with regard to the target values, the confidence in the threshold values varies from low to high.
The area of concern with respect to the uncertainty evaluation is the varying availability of the field-collected females for embryo analysis, and, thus possible inequality of the sample size between the stations/assessment units (Table 4). The statistical analyses underlying indicator evaluation employ bootstrapping to generate distributions for identical sample size and derive an estimate of the confidence interval. To decrease statistical uncertainty of the analysis, aggregation of the sampling stations into regions of similar bottom morphology, depth and trophy is recommended.
Thus, the overall confidence is considered to be moderate for this evaluation.
6 Drivers, Activities, and Pressures
The indicator is mainly sensitive to the effects of contaminants, i.e. trace metals and hydrophobic persistent organic contaminants (HOCs). A metadata analysis for Monoporeia affinis at 42 sites in polluted coastal areas was used to assess the correlation between malformations and industrial waste waters. A significant relationship was found between distance to industrial outlet and malformation rate (Reutgard et al. 2014), confirming earlier studies and linking malformations to in situ exposure (Elmgren et al. 1983, Sundelin and Eriksson 1998, Sundelin et al. 2008b) and genotoxicity assayed as DNA modifications in the amphipods (Gorokhova et al. 2021). The latter results implicate chemical exposure as the primary pressure for the reproductive aberrations, because non-chemical conditions, such as hypoxia and temperature, cannot drive such modifications.
Different embryo malformation types arise in the amphipods exposed to environmental contaminants. Moreover, some types of embryo aberrations were significantly associated with specific contaminant groups in the sediment (Löf et al. 2016). In particular, occurrence of females with embryo limb malformations was strongly related to elevated concentrations of Cd and PCBs, while females with membrane-damaged embryos occurred at high PAH concentrations. Also, frequency of embryos with arrested development was higher at elevated concentrations of PAHs and metals. Thus, these reproductive aberrations in M. affinis can serve as contaminant-specific indicators of PCB, PAH and heavy metal exposure in biological effect monitoring. Moreover, such aberrations as dead broods and dead eggs may indicate exposure to low oxygen concentrations during the oogenesis (Eriksson-Wiklund and Sundelin 2001, 2004). Whereas a combination of oxygen deficiency and contaminants has been observed to enhance contaminant toxicity (Gorokhova et al 2013), the oxygen deficiency as a sole factor does not give rise to malformed embryos. Food deficiency may also result in low fecundity and arrested development (Sundelin et al. 2008a), but not in other malformation types. More work is, therefore, needed to unambiguously link specific malformations to xenobiotic compounds or contaminant classes in various environmental setting.
Human activities that result in the production and release (accidental or via waste treatment) of hazardous substances are numerous, as are the drivers and societal behaviours behind these activities. Changing trends and approaches for production, disposal and use of hazardous substance containing products/materials can have a significant influence on their eventual release into the environment and therefore on the outcome of this indicator. Assembling the structures and approach to better understand drivers, activities and pressures in relation to hazardous substances is substantive piece of work needed for the future.
Table 6. Brief summary of relevant pressures and activities with relevance to the indicator.
| | General | MSFD Annex III, Table 2a |
| Strong link | The most important anthropogenic threat to malformed embryos of amphipods is exposure to contaminants in the sediment. | Substances, litter and energy
Input of other substances (e.g. synthetic substances, non-synthetic substances, radionuclides) – diffuse sources, point sources, atmospheric deposition, acute events |
| Weak link | Hypoxia combined to contaminants increase the incident of malformations |
7 Climate change and other factors
Climate change is predicted to have numerous impacts on the Baltic Sea ecosystem, as set out in the Climate Change in the Baltic Sea Fact Sheet (HELCOM and Baltic Earth, 2021). Elevated rainfall leading to runoff events or more terrestrial inputs to the Baltic Sea (especially coastal areas) may result in transfer of hazardous substances to the Baltic Sea marine environment. These additional inputs, or disturbance of settled contaminated materials, may result in further contact or increased bioavailability of hazardous substances, potentially influencing this indicator.
Although this indicator has been shown to be effective in specifically addressing the biological effects of hazardous substances future cumulative effects from climate change may also increase the pressure on components of the Baltic Sea ecosystem making them more susceptible to the existing or emerging contaminant pool.
8 Conclusions
The evaluation for the period 2016-2021 concluded that the Quark and the Western Gotland Basin have achieved good status, whereas the Bothnian Sea, Northern Baltic Proper, and the Gulf of Finland were not in good status. The deviations in frequencies of embryo malformations and of females carrying malformed embryos in the amphipod populations indicate reproductive and developmental toxicity to exceed acceptable levels in most of the evaluated areas. While no change in status is documented between the current and earlier evaluations, in their respective assessment units, a worsening situation can be identified from trends in the Western Gotland Basin that suggest a negative development of the reproductive health status in the amphipod populations.
8.1 Future work or improvements needed
At present, the embryo malformation indicator has not been evaluated for all assessment units in the Baltic Sea, partly due to the lack of monitoring in these areas. The validation of the applicability of the indicator and the determination of the threshold values are needed in the Åland Sea as well as much of the south-eastern, and southern Baltic Sea before the evaluation for these areas can be conducted. Using gammarids for this evaluation would make it possible to provide evaluation in the areas where M. affinis is not available.
In Monoporeia affinis populations exposed to contaminated sediments in situ, frequencies of different aberration types were found to be a function of the contaminant type. In particular, occurrence of females with embryo limb malformations was strongly related to elevated concentrations of Cd and PCBs, while females with membrane-damaged embryos occurred at high PAH concentrations. Also, frequency of embryos with arrested development was positively related to levels of PAHs and metals. Thus, these reproductive aberrations can serve as contaminant-specific indicators of PCB, PAH and heavy metal exposure in biological effect monitoring.
The integration of the contaminant-specific evaluation and relative frequencies for specific embryo malformations (Figure 2) would be necessary to establish a coherent evaluation for biological effects of contaminants in the Baltic Sea. Depending on the outcome of the integrated analysis of the chemical and biological data, the next step might include development of thresholds for specific malformation types. The latter would provide several additional dimensions to the multimetric embryo malformation indicator to increase its specificity toward particular contaminant types, while keeping the same concept.
9 Methodology
9.1 Scale of assessment
The indicator evaluation is done at HELCOM assessment unit Level 2, because no difference in the background values for the malformation rate between the coastal and the open sea areas was found. To evaluate the status of an assessment unit, a >50%-rule is applied, meaning that assessment unit is considered as being in good status when >50% stations with a region and >50% regions within a basin and >50% years within the assessment period are in good status. It should however be noted, that the contaminant load in sediments may vary significantly over relatively short distances and that the one-out-all-out approach may evaluate a large assessment unit as not in good status based on a station with heavily contaminated sediments. To provide a more balanced approach for such deviations and to account for a spatial variability, a region-based sampling design is recommended (Figure 8); however, it is also possible that an assessment unit is represented by a single region (e.g., the Quark) sharping this case, deviations from the BAC for a single station would be less likely to affect the evaluation outcome. As some natural variation in the malformation frequencies from year to year is expected, it is recommended that the status evaluation is conducted using data for at least 3 years.
As pollution pressure is distinctly different in different sub-basins, evaluations made by grouping neighbouring assessment units is not deemed possible. For example, the Bothnian Bay area cannot be evaluated using monitoring data from the Bothnian Sea; hence, as no monitoring is currently carried out in the Bothnian Bay, this sub-basin cannot be evaluated.
The assessment units are defined in the HELCOM Monitoring and Assessment Strategy Annex 4.
9.2 Methodology applied
To decrease statistical uncertainty of the analysis, a high number of sampling stations per assessment unit is recommended. Since sample size (i.e., number of gravid females available for the analysis) may have high temporal (year-to-year) and spatial (stations within assessment unit) variability, bootstrapping can be applied to control for the sample size. For each station, a sample size of 50 gravid females and about 1,500 embryos is the recommended sample size within the Swedish National Marine Monitoring Program for evaluating the proportion of malformed embryos in the population. Depending on the area of the assessment unit, distribution of sustained populations of the species in question, and heterogeneity of hydrography, several survey regions should be included, with several stations per region (Figure 8).
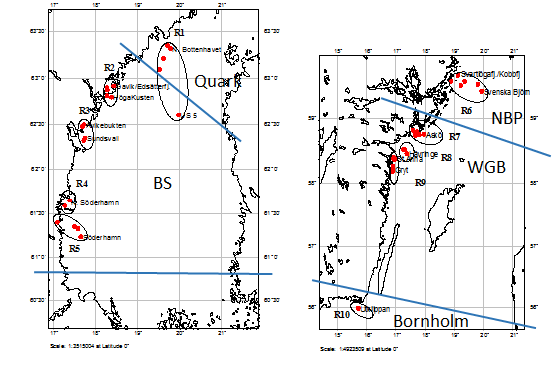
Figure 8. Sampling design employed in SNMMP for biological effect monitoring of the amphipod Monoporeia affinis. Red dots denote sampling stations and ellipses are the survey regions (R1 to R10) along the Swedish coast. Basins are abbreviated as in Results figure 1. All stations are located in areas free from pollution point sources and, therefore, the indicators values are expected to be within BAC. For the evaluation, a >50%-rule is applied.
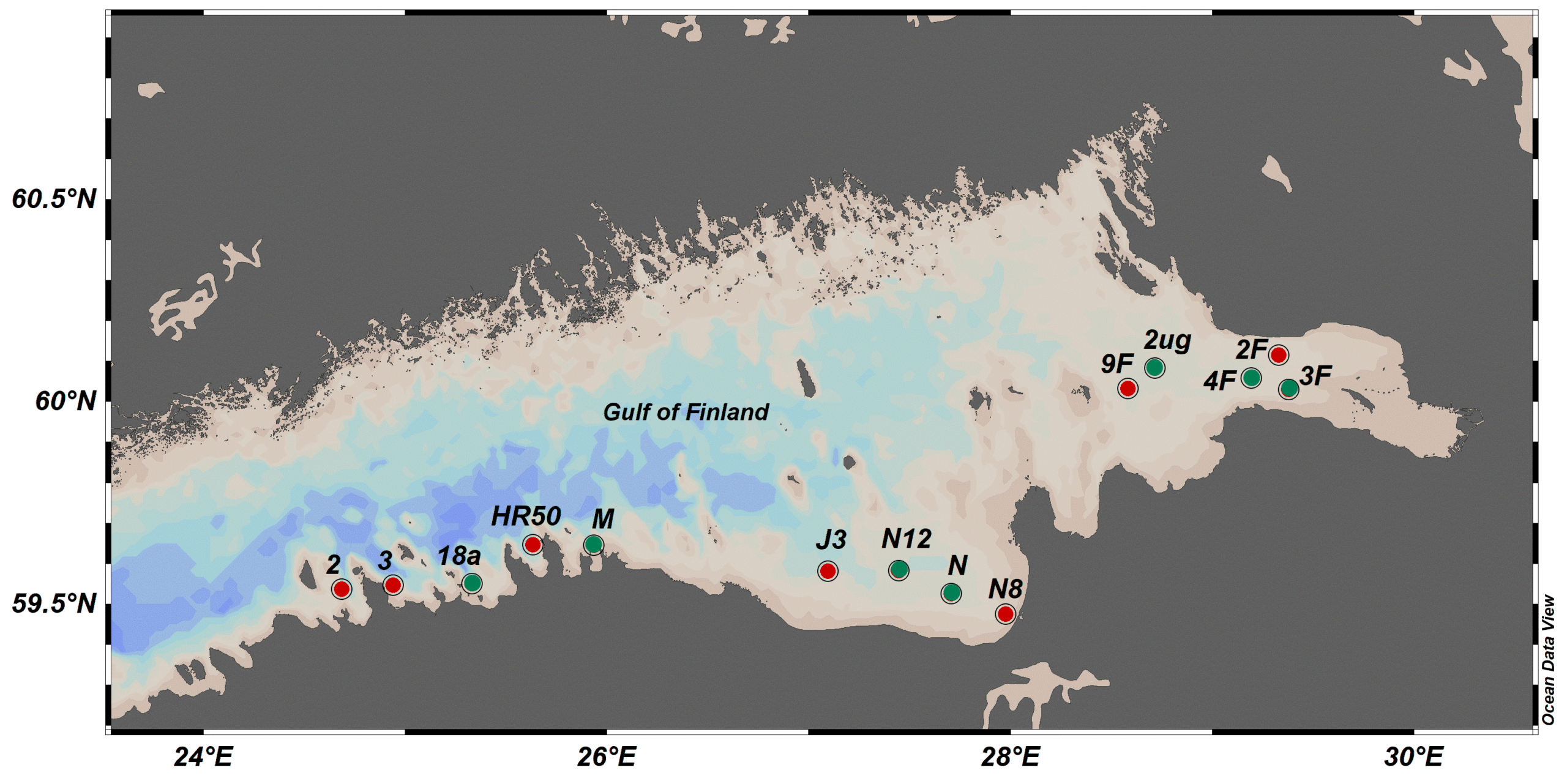
Figure 9. Sampling design employed in the Gulf of Finland for biological effect monitoring of the amphipod Monoporeia affinis. The reference stations (green) were used to derive threshold values; they were considered as reference sites based on the analysis of chemical contaminants in the sediment collected from the same stations. Data on the amphipod malformations from the red stations were added to conduct the evaluation for 2016-2021. No region-level aggregation was applied for the evaluation.
Evaluating whether an area is in good status using amphipods is done by:
- Detecting the number of malformed embryos in each brood by microscopic analysis and the number of females that carry more than 1 malformed embryo; see Table 2 in Löf et al. 2016 for the description of embryo malformations and the criteria for the classification of embryo pathologies;
- Calculating the percentage of malformed embryos and the number of females that carry more than one malformed embryo in the metapopulation (station); to keep the sample size (i.e. number of the females examined) constant, bootstrapping should be used;
- Combining the evaluation outcome for the region if several stations per region are used; otherwise, proceed with the station-specific values;
- Comparing the detected malformation rates for the station/region to the threshold values and concluding whether the frequency of malformed embryos and the frequency of females that carry more than one malformed embryo are below their respective threshold values.
Primary threshold values for Monoporeia affinis have been calculated using a subset of assessment units where monitoring data for this species were available (Figure 8, Figure 9). In areas where M. affinis does not occur in sufficient numbers, such as the Gdansk Basin and Arkona Basin, other amphipod species can be used, with corresponding secondary thresholds.
9.3 Monitoring and reporting requirements
Monitoring methodology
Monitoring of embryo malformation rates in the Contracting Parties of HELCOM is described on a general level in the HELCOM Monitoring Manual in the monitoring topic Biological effects of contaminants.
However, no specific sub-programme or HELCOM monitoring guidelines have so far been developed. As the information is not yet included in the HELCOM Monitoring Manual, a description of the main points of the guidelines applied nationally are presented in the indicator report. Monitoring guidelines are documented in detail in ‘Handboken för miljöövervakning’ (in Swedish) (eng. The handbook for environmental monitoring).
The amphipod Monoporeia affinis and the marine amphipod Pontoporeia femorata are included in the national monitoring program in Sweden (SNMMP). However, P. femorata has a very low abundance in the Bothnian Sea and the Quark. Collection of gravid females of the amphipods Monoporeia affinis and Pontoporeia femorata takes place in mid-to late January, when the embryos are in late developmental stages, which facilitates embryo analysis. To obtain a quantitative sample, a grab sampler (e.g. Van Veen) is used to collect amphipods inhabiting sediments. When amphipods occur at low abundances, a bottom sled is used to achieve greater sampling efficiency. To collect the sediment-dwelling amphipods, 5-and 1-mm sieves could be used depending on the size of the amphipods. For species producing several broods during the reproductive period, the sampling should preferably be carried out in the early mating period when specimens in the population demonstrate a more synchronous maturation than in the later part of the reproductive period. It is also possible to collect sediment and sexually maturing females and males in situ, to be incubated in aquaria allowing for mating and embryogenesis. In this case, the field-collected amphipods should be transported to the laboratory in ambient water/sediment and at temperature matching their natural habitat at the time of sampling. Many Baltic glacial relicts are stenotherm cold-water species and are particularly sensitive to temperature stress during the oogenesis (Eriksson Wiklund and Sundelin 2001).
The analysis of embryos is performed on living gravid females under a stereomicroscope. The frequency of malformed embryos of Monoporeia affinis has a comparatively low variation in pristine areas and 50 gravid females per station give sufficient statistical power. They are analysed for fecundity in terms of the number of eggs per female, the number of malformed, membrane-damaged embryos, dead embryos, and females with dead broods. For details see Sundelin et al. 2008 (Times no 41, http://ices.dk/publications/our-publications/Pages/-ICES-Techniques-in-Marine-Environmental-Sciences-.aspx). When conducting embryo analyses, several persons analyse the same brood to assess the accuracy of the determination. Normally there is 95-98 % agreement between the experts.
The amphipods used for the secondary threshold are G. tigrinus, G. fasciatus and P. robustoides. These species were used as bioindicators in survival tests within Russian Research Monitoring Programs by different institutions from the 1990s, and monitoring of amphipod reproduction started in 2009. This method is developed mainly by the Zoological Institute of the Russian Academy of Sciences. A preliminary evaluation of environmental health in the eastern Gulf of Finland was conducted at 12 coastal sites and is planned to continue. Collection of these amphipods is conducted from end of May to September by a grab or scuba diving. The field-collected living amphipods are transported carefully to the laboratory and analysed under a stereomicroscope. The amphipods are separated into four categories: juveniles, males, females I (without eggs in marsupium) and females II (gravid females, with eggs/embryos in marsupium). The determination of the reproductive status of the females is based on the presence and structure of brood plates (oostegites). The immature females have oostegites lacking setae. The gravid females are size-sorted in a 0.5 mm step and placed individually into Petri dishes; then the eggs are carefully teased out of the marsupium with pins or forceps. Only females carrying a closed marsupium are included in the analysis. The seven developmental stages of eggs (embryos) may be distinguished according to Weygoldt (1924) and Scadsheim (1982), cited in Pöckl (1993).
Current monitoring
ReproIND has been monitored in the SNMMP since 1994 at 14 stations in the Bothnian Sea and northern Baltic proper, in 2012 the program design was changed to include more stations to give a more comprehensive coverage of the Baltic. Monitoring of amphipod reproduction in the Gulf of Finland started in 2009 and is conducted by the Zoological Institute of the Russian Academy of Sciences. Estonian and Latvian scientists have also started to monitor Monoporeia affinis in the Gulf of Riga and gammarids in the coastal areas.
Description of optimal monitoring
Monitoring of embryo malformation rates should be carried out in all relevant HELCOM assessment units. Extending the spatial scope of the monitoring effort would increase the possibility of accurately evaluating the pressure from environmental contaminants on benthic animals in the Baltic Sea. To obtain a more comprehensive picture of the health status of the amphipods, it would be optimal to include also stations in the Bothnian Bay, the southern part of the Baltic, including Bornholm. A particularly alarming situation in the Hanö Bight has been found by the surveys conducted during the assessment period.
10 Data
The data and resulting data products (e.g. tables, figures and maps) available on the indicator web page can be used freely given that it is used appropriately and the source is cited.
Result: Reproductive disorders: malformed embryos of amphipods
Data: Reproductive disorders: malformed embryos of amphipods
11 Contributors
Elena Gorokhova1, Brita Sundelin1, Natalia Kolesova2, Evita Strode3, and Nadezhda Berezina4
1 Department of Environmental Science, Stockholm University
2 Department of Marine Systems, Tallinn University of Technology, Estonia
3 Latvian Institute of Aquatic Ecology, Latvia
4 Zoological Institute, Russian Academy of Sciences, Russia
HELCOM Secretariat: Jannica Haldin, Owen Rowe
12 Archive
This version of the HELCOM core indicator report was published in April 2023:
The current version of this indicator (including as a PDF) can be found on the HELCOM indicator web page.
Earlier indicator reports are available at:
Reproductive disorders malformed embryos of amphipods HELCOM supplementary indicator 2018 (pdf)
HOLAS II component – Core indicator report – web-based version July 2017 (pdf)
13 References
Aneer G (1975) Composition of food of the Baltic herring (Clupea harengus v. membrans L.) fourhorn sculpin (Myoxocephalus quadricornis L.) and eel-pout (Zoarces viviparus L.) from deep soft bottom trawling in the Askö – Landsort area during two consecutive years. Meerentutkimuslait. Julk/Havsforskningsinst Skr 239: 146-154
Ankar S, Sigvaldadottir E (1981) On the food composition of Halicryptus spinulosus von Siebold. Ophelia 20: 45-51
Bacela K, Konopacka A (2005) The life history of Pontogammarus robustoides, an alien amphipod species in Polish waters. Journal of Crustacean biology 25:190–195.
Berezina NA (2007) Food spectra and consumption rates of four amphipod species from the North-West of Russia. Fundamental and Applied Limnology/ Archiv fur Hydrobiologie 168 (4):317–326.
Berezina NA, Petryashev VV, Razinkovas A, Lesutiene J (2011) Alien malacostraca in the eastern Baltic Sea: pathways and consequences. In: Galil BS, Clark PF, Carlton JT (eds), In the Wrong Place – Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature – Springer Series in Invasion Ecology 6: 301–322.
Berezina NA, Gubelit YI, Polyak YM, Sharov AN, Kudryavtseva VA, Lubimtsev VA, Petukhov VA, Shigaeva TD (2017) An integrated approach to the assessment of the eastern Gulf of Finland health: A case study of coastal habitats. Journal of Marine Systems. 171: 159–171.
Davies, I. M. and Vethaak, A. D. 2012. Integrated marine environmental monitoring of chemicals and their effects. ICES Cooperative Research Report No. 315. 277 pp.
Elmgren, R., S. Hansson, U. Larsson, B. Sundelin and P. Boehm. (1983). The “Tsesis” oil spill: Acute and long-term impact on the benthos. Mar. Biol. 73, 51-65.
Eriksson, A-K., Sundelin, B., Broman, D., Näf, C (1996). Effects on Monoporeia affinis of HPLC-fractionated extracts of bottom sediments from a pulp mill recipient. In: Environmental fate and effects of pulp and paper mill effluents. Servos et al (eds) p 69-78, St Lucie Press Florida
Eriksson-Wiklund, A-K. B. Sundelin. (2001). Impaired reproduction of the amphipods Monoporeia affinis and Pontoporeia femorata as a result of moderate hypoxia and increased temperature Mar. Ecol. Prog. Ser. 171:165-180.
Eriksson Wiklund AK, Sundelin B (2004). Biomarker sensitivity to temperature and hypoxia- a seven year field study. Mar Ecol Prog Ser274: 209-214.
Eriksson Wiklund A-K, Sundelin B, Broman D. (2005). Toxicity evaluation by using intact sediments and sediment extracts, Mar Poll Bull 50 (6): 660-667
Eriksson Wiklund, Sundelin B, Rosa R (2008). Population decline of the amphipod Monoporeia affinis in Northern Europe, consequence of food shortage and competition. J Exp Mar Biol Ecol, 367; 81-90.
Gorokhova E, Löf M, Reutgard M, Lindström M, Sundelin B (2013). Exposure to contaminants exacerbates oxidative stress in amphipod Monoporeia affinis subjected to fluctuating hypoxia. Aquatic Toxicology 127:46-53
Gorokhova, E., Martella, G., Motwani, N. H., Tretyakova, N. Y., Sundelin, B., and Motwani, H. V. (2020). DNA epigenetic marks are linked to embryo aberrations in amphipods. Scientific Reports 10. doi: 10.1038/s41598-020-57465-1
HELCOM (2014) BASE project 2012-2014: Preparation of biodiversity and hazardous substances indicators with targets that reflect good environmental status for HELCOM (including the HELCOM CORESET project) and improvement of Russian capacity to participate in operationalization of those indicators. 2014 Baltic Marine Environment Protection Commission HELCOM. 264 p.
HELCOM and Baltic Earth (2021): Climate Change in the Baltic Sea Fact Sheet. https://helcom.fi/wp-content/uploads/2021/09/Baltic-Sea-Climate-Change-Fact-Sheet-2021.pdf
ICES (2021) ICES VIEWPOINT: Assessment of the Biological Effects of Chemical Pollution for Better Management of the Marine Environment. https://doi.org/10.17895/ICES.ADVICE.8478
Jacobson, T. Prevodnik A. and Sundelin B (2008). Combined effects of temperature and a pesticide on the Baltic amphipod Monoporeia affinis. Aquatic Biology 1: 269-276.
Jacobson T, Sundelin B.(2006). Reproduction effects of the endocrine disruptor fenarimol on a Baltic amphipod, Monoporeia affinis. Environ. Tox. Chem 25:1126-1131
Jacobson T, Holmström K, Yang G, Ford AT, Berger U Sundelin B (2010). Perfluoroctane sulfonate accumulation and parasite infestation in a field population of the amphipod Monoporeia affinis after microcosm exposure. Aquatic Toxicology 98 99–106
Jacobson T, Yang G, Ford AT, Sundelin B (2011). Low dose TBT exposure decreases amphipod immunocompetence and reproductive fitness. Aquatic Toxicology 101:72-77.
Kuparinen J, K. Leonardsson J. Mattila, and J. Wikner. (1996). Food web structure and function in the Gulf of Bothnia, the Baltic Sea. Ambio:13–21.
Lehtonen K, Sundelin B, Lang T, Strand J (2014). Development of tools for integrated monitoring and assessment of hazardous substances and their biological effects in the Baltic Sea. Ambio 43:69–81.
Lindström M (1992) The migration behaviour of the amphipod Pontoporeia affinis (Lindström). Walter and Andrée de Nottbeck foundation scientific reports no 7 Dissertation, University of Helsinki.
Löf M, Sundelin B, Bandh C, Gorokhova E (2016), Embryo aberrations in the amphipod Monoporeia affinis as indicator of toxic pollutants in sediment, a field evaluation. Ecol Indicator 60:18-30
Panov VE, Berezina NA (2002) Invasion history, biology and impacts of the Baikalian amphipod Gmelinoides fasciatus (Stebb.). In Leppäkoski E., Olenin S. & Gollasch S. (eds.), Invasive Aquatic Species of Europe. Kluwer Publ., Dordrecht:96–103.
Pöckl M. (1993) Reproductive potential and lifetime potential fecundity of the freshwater amphipods Gammarus fossarum and G. roeseli in Austrian streams and rivers. Freshwater Biology 30: 73–91.
Raymond, C., Gorokhova, E., Karlson, A.M., 2021. Polycylic aromatic hydrocarbons have adverse effects on benthic communities in the Baltic Sea: implications for environmental status assessment. Front. Environ. Sci. 9. https://doi.org/10.3389/fenvs.2021.624658
Reutgard M, Eriksson Wiklund A-K, Breitholtz M, Sundelin B. (2014). Embryo development of the benthic amphipod Monoporeia affinis as atool for monitoring and assessment of biological effects ofcontaminants in the field: A meta-analysis. Ecological indicator 36:483-490.Shiedek, D., Sundelin, B., Readman, J.W., McDonald, R.W. (2007). Interactions between climate change and contaminants, a review. Mar Poll Bull 54: 845-856.
Sparrevik E, Leonardsson K (1995) Effects of large Saduria entomon (Isopoda) on spatial distribution of their small S.entmon and Monoporeia affinis (Amphipoda) prey. Oecologia 101: 177-184
Strode E, Jansons M, Purina I, Balode M, Berezina NA (2017) Sediment quality assessment using survival and embryo malformation tests in amphipod crustaceans: The Gulf of Riga, Baltic Sea as case study. Journal of Marine Systems. 172: 93–103. Sundelin, B. (1983). Effects of cadmium on Pontoporeia affinis (Crustacea: Amphipoda) in laboratory soft-bottom microcosms. Mar. Biol. 74, 203-212.
Sundelin, B. (1984). Single and combined effects of lead and cadmium on Pontoporeia affinis (Crustacea: Amphipoda) in laboratory soft-bottom microcosms. In: Ecotoxicological testing for the marine environment. G. Persoone, E. Jaspers, and C. Claus (Eds). State Univ. Ghent and Inst. Mar. Scient. Res., Bredene, Belgium. Vol. 2. 588 p.
Sundelin, B. (1988). Effects of sulphate pulp mill effluents on soft-bottom organisms – a microcosm study. Wat. Sci. Tech. Vol. 20, No. 2, pp. 175-177.
Sundelin, B., A-K. Eriksson (1998). Malformations in embryos of the deposit-feeding amphipod Monoporeia affinis in the Baltic Sea. Mar. Ecol. Prog. Ser. 171: 165-180.
Sundelin, B., Ryk, L, Malmberg, G. (2000) Effects on the sexual maturation of the sediment-living amphipod Monoporeia affinis. Environ. Toxicol 15: 5, 518-526.
Sundelin, B., A-K. Eriksson. (2001). Mobility and Bioavailability of Trace Metals in Sulfidic Coastal Sediments. Environ Toxicol Chem 20: (4) 748-756.
Sundelin, Rosa, R., Eriksson Wiklund, A-K (2008a). Reproduction disorders in a benthic amphipod, Monoporeia affinis, an effect of low food quality and availability. Aquatic Biology, 2:179-190.
Sundelin B., Eriksson Wiklund A-K, Ford A (2008b). The use of embryo aberrations in amphipod crustaceans for measuring effects of environmental stressors. ICES Techniques in Marine Environmental Sciences no 41 (TIMES).
14 Other relevant resources
No additional information is required.