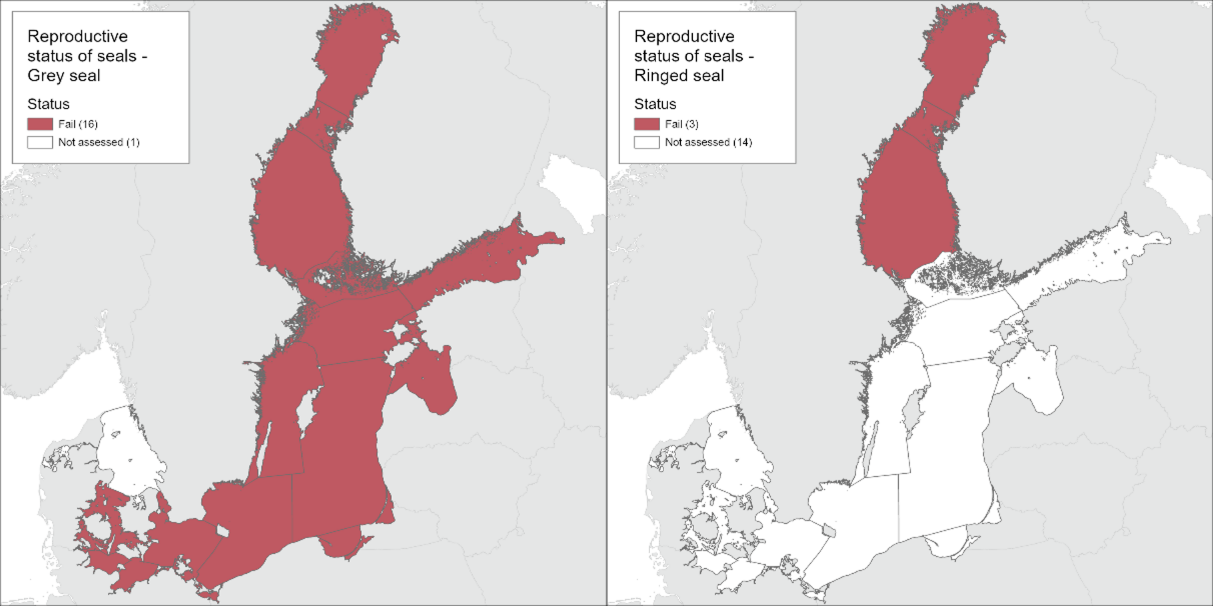
 Seal reproduction
Seal reproduction
2.1 Ecological relevance
Marine mammals are top predators in the marine ecosystem and as such are good indicators of the state of food webs. Due to their top position in the food web and dependency on fat for energy, insulation and lactation, they are at risk for accumulation of fat-soluble hazardous substances such as organic pollutants. Marine mammals are also affected by human influences that cause stress and disturbance such as hunting, by-catch, over-fishing, disturbance and noise pollution (all related to human activities at sea). The effect of algal toxins on seal reproductive health is so far unknown. The vulnerability of seals to these pressures makes them overall good indicators for measuring the environmental status of ecosystems.
Distributions of different seal species during feeding and annual migrations encompass the entire Baltic Sea. Monitoring of relevant reproductive rate parameters occurs in all countries where stranded, by-caught or hunted seals are collected.
The reproductive rate provides important information both for the population in general and on reproductive health in the population in particular. An adult female seal bears at most one pup annually in healthy growing seal populations. The mean values of fecundity for entire populations will always be lower than the theoretical maximum for an individual, also for populations which live under favourable conditions. Chance events such as failed fertilization or early abortions reduce annual pregnancy rates. Mean pregnancy rates rarely reach 0.96 in samples of reasonable sizes in American (Boulva & McLaren 1979; Bigg 1969), and European harbour seals (Heide-Jørgensen et al. 1992) in age classes >4 years of age. Maximum life span is about 35-45 years in Baltic seal species (e.g. Heide-Jørgensen et al. 1992). Another factor that will decrease mean pregnancy rates is senescence (Heide-Jorgensen et al. 1992), however due to annual mortality rates, only a small fraction of the population becomes older than about 24 years old. Further, extrinsic factors will reduce pregnancy rates. In evaluating changes in mean pregnancy rate among years in this core indicator, it is important to separate the causes into (1) natural decline due to density dependent effects and (2) anthropogenic effects from environmental pollution. The HELCOM core indicator ‘Population trends and abundance of seals’ will signal when the populations reach carrying capacity. But at population abundances below carrying capacity, a change in pregnancy rate can be an early warning of unwanted changes in the ecosystem.
2.1.1 Natural decline in fertility due to limited food supply
As seal populations approach carrying capacity and food limitation becomes an issue, body growth rate in sub-adult seals declines and the age at sexual maturation is delayed. In poor nutritive conditions, age at sexual maturity in phocid seals can be delayed up to three or four years (Kjellqvist et al. 1995; Harding & Härkönen 1999). Other stressors such as infectious disease and stress can also delay sexual maturity. Another response to poor nutritive conditions is so called ‘year skipping’, i.e. the female does not become pregnant when her fat stores are too low (Kjellqvist et al. 1995). Seals have delayed implantation and the fertilized egg does not attach to the uterine wall unless the female is well fed. Decreased pregnancy rate due to food shortage at carrying capacity is thus a natural phenomenon and shall not be confused with reproductive failure caused by disease or xenobiotics.
2.1.2 Reproductive failure caused by disease or xenobiotics
The Baltic ringed and grey seal populations became the main subjects in the PCB contamination. The mean level of PCB in seals from the northern Baltic Proper was about 450 parts per million (PPM) lipid in the early 1970s, which eventually declined to considerably lower values in accordance with lower concentrations in their prey (Jensen et al. 1969; Olsson 1977; Bignert et al. 1998). A sample of 225 adult ringed seal females revealed an alarmingly low pregnancy rate of 30% which dropped further to 20% during the period 1973-1979 (Helle 1980). The low reproductive rates were largely explained by occlusions in the uterine horns. The prevalence of this pathological change increased from 35% to 59% during the same time period (Helle 1980). The occlusions caused permanent sterility in ringed seals and the frequency of occlusions also increased with the age of the animals (Helle 1979; 1980). Also in grey seals, severe reproductive disturbances were documented (Bergman & Olsson 1986; Bergman 1999). An underlying cause of some of the toxic effects of PCBs may be alterations in hormonal levels (Bäcklin et al. 2003). Experiments carried out on the American mink (Neovison vison) showed that the early formation of the placenta is disrupted in animals exposed to PCBs, which leads to the death of the foetus (Bäcklin et al. 2003).
In populations of harbour seals, concentrations of PCBs vary with the level of industrialization and the extent of water exchange of different sea regions. This is demonstrated by mean values of concentrations of different PCB fractions in harbour seals in the Atlantic, where Icelandic harbour seals have the lowest concentrations of about 1.5-5.0 PPM lipid, while seals in the heavily industrialized and enclosed St. Lawrence Estuary show concentrations of about 17.1 PPM (Safe 1984). The harbour seals in the Baltic Sea and Wadden Sea had mean concentrations of 85 PPM lipid (Bernt et al. 1999) in the late 1970s. The effects of high levels of PCBs are generally very difficult to quantify. One reason is that levels of PCBs vary substantially depending on which part of the season, which age groups, individuals and which parts of the body are sampled (Safe 1984; Bignert et al. 1993). However, a controlled feeding experiment revealed lowered pregnancy rates in captive seals fed with Baltic herring compared to the control group that got North Sea herring (Reijnders 1986). The most likely candidate responsible for the former low gynaecological health among Baltic seals was high concentrations of PCB (Helle 1979; Bredhult et al. 2008; ). Levels in the Wadden Sea harbour seal populations are still quiet high (Siebert et al. 2012), nevertheless the populations have recovered very quickly after each die-off.
In 2008-2009, the pregnancy rate was 88% in 4-20 years old grey seal females hunted in the Bothnian Sea and the Baltic Proper. The last case of uterine obstruction in grey seals investigated in Sweden was seen in 1993 (Bergman 1999). And in 2009, one unilateral occlusion was seen in a 13-year old female grey seal in Finland. In the 2000s, about 20% of examined Baltic ringed seals still suffered from uterine obstructions, which likely explain the 68% pregnancy rate in ringed seals in 2001-2009, which is lower than “normal” (Helle et al. 2005; Kunnasranta 2010). After the year 2000 there are 62 females which are at least four years old (data from Finland and Sweden), and 8.1% of these had occusions. The last observed case is from 2011. There are no observations or reports of uterine obstructions in Baltic harbour seals or harbour porpoises.
It is important to distinguish between pregnancy rate, birth rate, pup production (= pups that survive until weaning), and the role of pregnancy/birth rate rate for the population growth rate. Even if a female weans her pup successfully, a study on individually branded harbour seals showed a delayed response to poor nutritive conditions (Härkönen & Harding 2001; Harding et al. 2005). Winter survival in the young of the year was highly dependent on the autumn weight. Consequently, pregnancy/birth rate is an important indicator of status of the population, but in evaluations for population consequences also other information is needed, the new Seal Health Indicator aims to assess the causes behind shifting trends in pregnancy rates.
2.2 Policy relevance
Table 1: Policy relevance of indicator.
| Baltic Sea Action Plan (BSAP) | Marine Strategy Framework Directive (MSFD) | |
| Fundamental link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
|
| Complementary link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
Segment: Hazardous substances and litter goal Goal: “Baltic Sea unaffected by hazardous substances and litter”
|
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
Descriptor 1 Species groups of birds, mammals, reptiles, fish and cephalopods.
Descriptor 4 Ecosystems, including food webs.
Descriptor 8 Concentrations of contaminants are at levels not giving rise to pollution effects.
|
| Other relevant legislation: | In some Contracting Parties also EU Water Framework Directive – Chemical quality, Habitats Directive | |
2.3 Relevance for other assessments
The core indicator on reproductive status of seals addresses the 2021 Baltic Sea Action Plan’s (BSAP) (HELCOM 2021) Biodiversity segment’s ecological objectives ‘Viable populations of all native species’ and ‘Functional, healthy and resilient food webs’. The core indicator is relevant to the following specific BSAP action:
- B19: By 2023 finalise and implement national or local conservation and/or management plans for grey seals.
- B20 By 2023 finalise and implement national conservation and/or management plans for ringed seals.
- B21 By 2025 protect the ringed seal in the Gulf of Finland, including to significantly reduce by-catch and to improve the understanding of the other direct threats on the seals, and urge transboundary co-operation between Estonia, Finland and Russia to support achieving a viable population of ringed seals in the Gulf.
- ‘B23: By 2025 develop, and by 2027 implement, and enforce compliance with ecologically relevant conservation plans or other relevant programmes or measures, limiting direct and indirect pressures stemming from human activities for threatened and declining species. These will include joint or regionally agreed conservation measures for migrating species.
The HELCOM Recommendation 27/28-2 ‘Conservation of seals in the Baltic Sea area’ outlines the conservation goals, which the indicator threshold value is based on. The explicit long-term objectives of management plans to be elaborated are: Natural Abundance, Natural Distribution, and a health status that ensures the persistence of marine mammals in the Baltic.
The results are utilised in the HELCOM Biodiversity integrated assessment (BEAT tool) to support an overall evaluation of marine mammals.
The core indicator also addresses the following qualitative descriptors of the MSFD for determining good environmental status (European Commission 2008):
Descriptor 1: ‘Biological diversity is maintained. The quality and occurrence of habitats and the distribution and abundance of species are in line with prevailing physiographic, geographic and climatic conditions’
Descriptor 4: ‘All elements of the marine food webs, to the extent that they are known, occur at normal abundance and diversity and levels capable of ensuring the long-term abundance of the species and the retention of their full reproductive capacity’
Descriptor 8: ‘Concentrations of contaminants are at levels not giving rise to pollution effects’
Descriptor 10: ‘Properties and quantities of marine litter do not cause harm to the coastal and marine environment’ and
Descriptor 11: ‘Introduction of energy, including underwater noise, is at levels that do not adversely affect the marine environment’
and the following criteria of the Commission Decision on GES criteria (2017):
- D1C3 Population demographic characteristics of the species
- D1C2: The population abundance of the species
- D1C4: The species distributional range
- D4C4: Productivity of the trophic guild
- D8C2: The health of species and the condition of habitats are not adversely affected due to contaminants
Marine mammals were recognized by the MSFD Task Group 1 as a group to be assessed.
In some Contracting Parties the indicator also has potential relevance for implementation of the EU Water Framework Directive (WFD) (Chemical quality) (European Commission 2000) and Habitats Directive (European Commission 1992). The WFD includes status categories for coastal waters as well as environmental and ecological objectives, whereas the EU Habitats Directive specifically states that long-term management objectives should not be influenced by socio-economic considerations, although they may be considered during the implementation of management programmes provided the long-term objectives are not compromised. All seals in Europe are also listed under the EU Habitats Directive Annex II, and member countries are subsequently obliged to monitor the status of seal populations.
Good status for this indicator is achieved when the aggregated pregnancy ratio for annual gestation rate and rate of postpartum pregnancy signs achieve the threshold value of 90%. The initial evaluation addressed the aggregated variable to determine if it meets the level of at least 90% – for five years and older harbour seals and six years and older grey and ringed seals (Table 2).
Table 2. Species specific threshold values for seal species showing increasing populations (i.e. not currently at carrying capacity) in the Baltic Sea, as agreed by HELCOM HOD 48-2015 (outcome para 3.63, Annex 4).
| Species |
Threshold value Age class [year] |
Threshold value Pregnancy rate |
|
| Grey seal | ≥6 | 90% | |
| Ringed seal (tentative) | ≥6 | 90% | |
| Harbour seal | ≥5 | 90% |
3.1 Setting the threshold value(s)
The concept for defining threshold values for reproductive rates of seals is derived from the general management principle in the HELCOM Recommendation 27/28-2, which states that the population size is to be managed with the long-term objective of allowing seal populations to recover towards carrying capacity levels. The Recommendation further states that the long-term goal is to reach a health status that ensures the future persistence of marine mammals in the Baltic. Reproductive rate is an important aspect of population status, affecting population growth rate.
A modern baseline approach is applied for establishing the threshold value for all species of seals included in this indicator, using 1992 data as a baseline, since pristine conditions are unknown. The modern baseline is based on the first available data, and data on reproductive rates from populations with minimal impacts from human activities are used in this indicator.
Pregnancy rate is measured as the proportion of 5/6–24-year-old females, depending on the seal species, with an embryo or foetus during the pregnancy period (post-implantation period). Postpartum pregnancy rate is calculated from the pre-implantation sample as the proportion of 6/7–25-year-old females with post-partum signs, i.e. a corpus albicans/placental scar.
3.1.1 Grey seals
Pregnancy rate is measured as the presence of an embryo/foetus in the pregnancy period in 6–24 year-old (or ≥ 6 yr) seals. Birth rate is calculated from the pre-implantation sample as the proportion of 7–25-year-old (or ≥ 7 yr) females with a corpus albicans/placental scar. The reason for using the age interval 6-24 years is that estimated age-specific birth rates increase steeply from the age of four to six (Hamill & Gosselin 1995). The birth rates for six-year old females in the Northwest Atlantic, British, Norwegian and Baltic populations ranged between 60-91%. In a sample of 526 female grey seals from the Northwest Atlantic, pregnancy rates were estimated from the presence/absence of a foetus. The pregnancy rate for the Northwest Atlantic population was relatively stable at about 90% after the age of six (Hamill & Gosselin 1995; Harding et al. 2007). In the Baltic grey seal population, the pregnancy rate was 88% in 4–20-year old females in 2008–2009 (Figure 2). Thus, a pregnancy rate of 88% pregnancy seems to be normal in 4–20-year old Baltic grey seals in an increasing population (Figure 1 and Figure 2). This rate is also close to the pregnancy rate of Northwest Atlantic grey seals older than five years. The pregnancy rate for the 4–5-year old individuals was 65% and for the 6–20-year old individuals it was 95.5% among hunted and by-caught grey seals in 2002–2009 in Sweden (Figure 3).
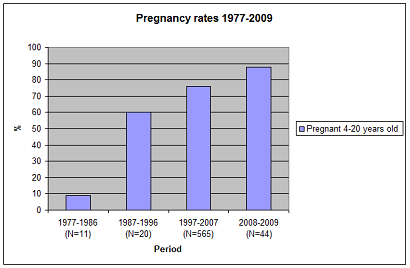
Figure 2. Pregnancy rate in 4–20-year old female Baltic grey seals (August to March). Finnish data for inferred birth rates is included in the period 1997–2007, in addition to Swedish pregnancy rate data.
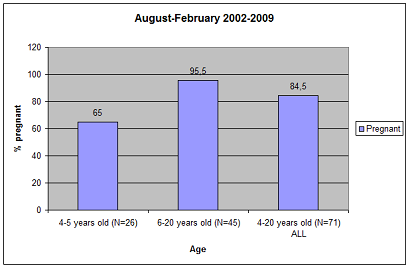
Figure 3. Pregnancy rate in 4-6 year-old females (first column), 6–20 year-olds (second column), and all age classes 4-20. Based on by-caught and hunted seals during 2002–2009.
The status evaluation should therefore be based on females six years or older (for pregnancy rate) to avoid effects from young females with late sexual maturity. Consequently, threshold values should be based on material sampled from age classes 6–24 for pregnancy rate and 7–25-year-old females for birth rate.
3.1.2 Ringed seals
Life history data of ringed seals is similar to grey seals (Harding et al. 2007), which would imply that the threshold value for ringed seals should be similar to that of grey seals. The threshold value of 90% is used also for this species in the absence of species specific information. Age classes to be included in the analysis should encompass six years and older. In the literature, pregnancy rates in different Artic populations seem to vary between 62-85% (Smith 1970). A recent study in Svalbard reported a gestation rate of 71% (Andersen et al. 2021).
Historically the annual number of investigated 6-20-year old Baltic female ringed seals during the pregnancy period has been very small. Figure 4 shows the pregnancy rate of a total number of 19 ringed seals examined during 1981-2009. The pregnancy rate in ringed seals was 68% in 2001-2009, but the sample size is confined to 9 animals.
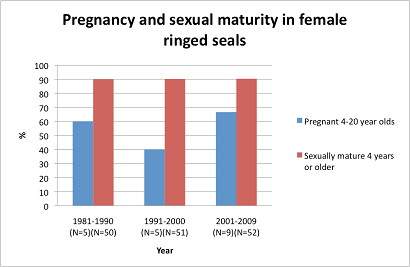
Figure 4. The prevalence of pregnant females (blue columns) sampled in the implantation period August to February (Kunnasranta 2010). Proportion of sexually mature (red columns) encompass females with presence of Corpus luteum (4 years or older) sampled year round in Finland and Sweden. Sample sizes must be increased before evaluations of status can be performed.
3.1.3 Harbour seals
The harbour seal historical pregnancy rates are based on samples from Danish and Swedish sampling programs in the Kattegat in 1988. When evaluating the threshold value at 90%, the age classes to be included are females of five years and older.
Large data sets were collected during the 1988 and 2002 phocine distemper virus (PDV) epidemics and 2014 influenza die-off that killed thousands of harbour seals. Pregnancy rates were determined either by signs of late abortions or the presence of pregnancy indicators (Heide-Jorgensen & Härkönen 1992). The pregnancy rate was found to be 94% in the 59 females older than 5 years that were sampled, and three of four females that were older than 25 years and senescent. This dataset can be used to establish a threshold value, and there are many samples available from the 2002 PDV epidemic as well as from later years in Sweden, stored at the Swedish Museum of Natural History. However, most of is the samples are from the Kattegat, and only few are available from the Southern Baltic Sea and the Kalmarsund area.
The results of the indicator evaluation that underpin the key message map and information are provided below.
4.1 Status evaluation
4.1.1 Grey seal
The grey seal did not achieve good status in the Baltic Sea with regard to reproductive status during assessment period 2016-2021 (Figure 5). The pregnancy rate (aggregated ratio) reached the threshold value in 2017 and in 2018, but on average across the current assessment period the threshold value of 90% was not achieved. Current pooled data from Finland and Sweden show that pregnancy rate is 87% (SE = 2.8%). The postpartum signs rate based on the presence of placental scars were used in the evaluation as that is in theory the most reliable postpartum sign. In Table 2 an alternative method, using presence of a corpus albicans (CA) if placental scars were not evaluated is presented, showing consistently lower reproductive rates.
Samples currently used in this evaluation are predominantly from the northern Baltic Sea, though they also include Swedish data from the southern Baltic Sea.
Figure 5. Baltic grey seal is not in good status with regard to reproductive rate.
Table 3. Grey seal pregnancy rate during 2016-2021, number of included seals in parentheses
| Year | Aggregated ratea | Standard error | Gestation rate (visible foetus in gestation period) | Postpartum signs rate – placental scar only | Postpartum signs rate, – placental scar OR CAb |
| 2016 | 85% (20) | 0.080 | 87% (15) | 80% (5) | 67% (6) |
| 2017 | 93% (28) | 0.049 | 94% (17) | 91% (11) | 79% (14) |
| 2018 | 95% (22) | 0.044 | 89% (9) | 100% (13) | 79% (19) |
| 2019 | 76% (25) | 0.085 | 72% (18) | 86% (7) | 78% (9) |
| 2020 | 89% (28) | 0.058 | 87% (23) | 100% (5) | 88% (8) |
| 2021 | 82% (17) | 0.092 | 82% (17) |
|
|
| Total | 87% (140) | 0.028 | 85% (99) | 93% (41) | 79% (56) |
a Combined gestation rate and postpartum signs rate using placental scars only
b Based on placental scars, but when placental scar evaluation was missing the presence of a CA (corpus albicans) was used for determining postpartum
4.1.1 Ringed seal
The ringed seal in the Bothnian Bay did not achieve good status with regard to reproductive status during 2016-2021 (Figure 6). The pregnancy rate (aggregated ratio) seems to have been relatively high in a limited sample collected during 2020-2021, but across the assessment period, the threshold value of 90% was not achieved. Current pooled data from Finland and Sweden show that pregnancy rate is 82% (SE = 0.034). The postpartum signs rate based on the presence of placental scars were used in the evaluation as that is in theory the most reliable postpartum sign. In Table 4 an alternative method, using presence of an ovarian CA if placental scars were not evaluated is presented, showing slightly lower reproductive rates.
Previous evaluations of ringed seal reproductive status have been hampered by low sample sizes and has indicated low reproductive rates, however, has also indicate an increasing trend (HELCOM 2018). During 2016-2021, the sample size in the Bothnian Bay increased to n=129 and an evaluation was considered possible for this assessment unit. There are no available data on reproductive rate for the assessment unit Southwestern Archipelago Sea, Gulf of Finland and Gulf of Riga.
Figure 6. Ringed seal is not in good status with regard to reproductive rate.
Table 4. Ringed seal pregnancy rate during 2016-2021, numbers in parentheses
| Aggregated ratea | Standard error | Gestation rate (visible foetus in gestation period) | Postpartum signs rate – placental scar only | Postpartum signs rate, – placental scar OR CAb | |
| 2016 | 69% (13) | 0.128 | 80% (5) | 62% (8) | 67% (9) |
| 2017 | 90% (20) | 0.067 | 100% (1) | 89% (19) | 81% (21) |
| 2018 | 84% (27) | 0.061 | 78% (9) | 86% (28) | 78% (32) |
| 2019 | 76% (38) | 0.069 | 75% (4) | 76% (34) | 70% (37) |
| 2020 | 90% (20) | 0.067 | 100% (5) | 87% (15) | 78% (65) |
| 2021 | 100% (1) | – | 100% (1) |
|
|
| Total | 82% (129) | 0.034 | 84% (25) | 82% (104) | 76% (164) |
a Combined gestation rate and postpartum signs rate using placental scars only
b Based on placental scars, but when placental scar evaluation was missing the presence of a CA (corpus albicans) was used for determining postpartum
4.1.1 Harbour seal
The threshold value is set at 90% also for harbour seals, but no evaluation could be conducted at this time due to data constraints. The sample size was too low and age determinations were missing for parts of the data.
4.2 Trends
In both grey seal and ringed seal the overall pregnancy rates have significantly increased during the last 15 years (Figure 7), although the ringed seal reproductive status seems to have improved at a slower rate compared to the grey seal.
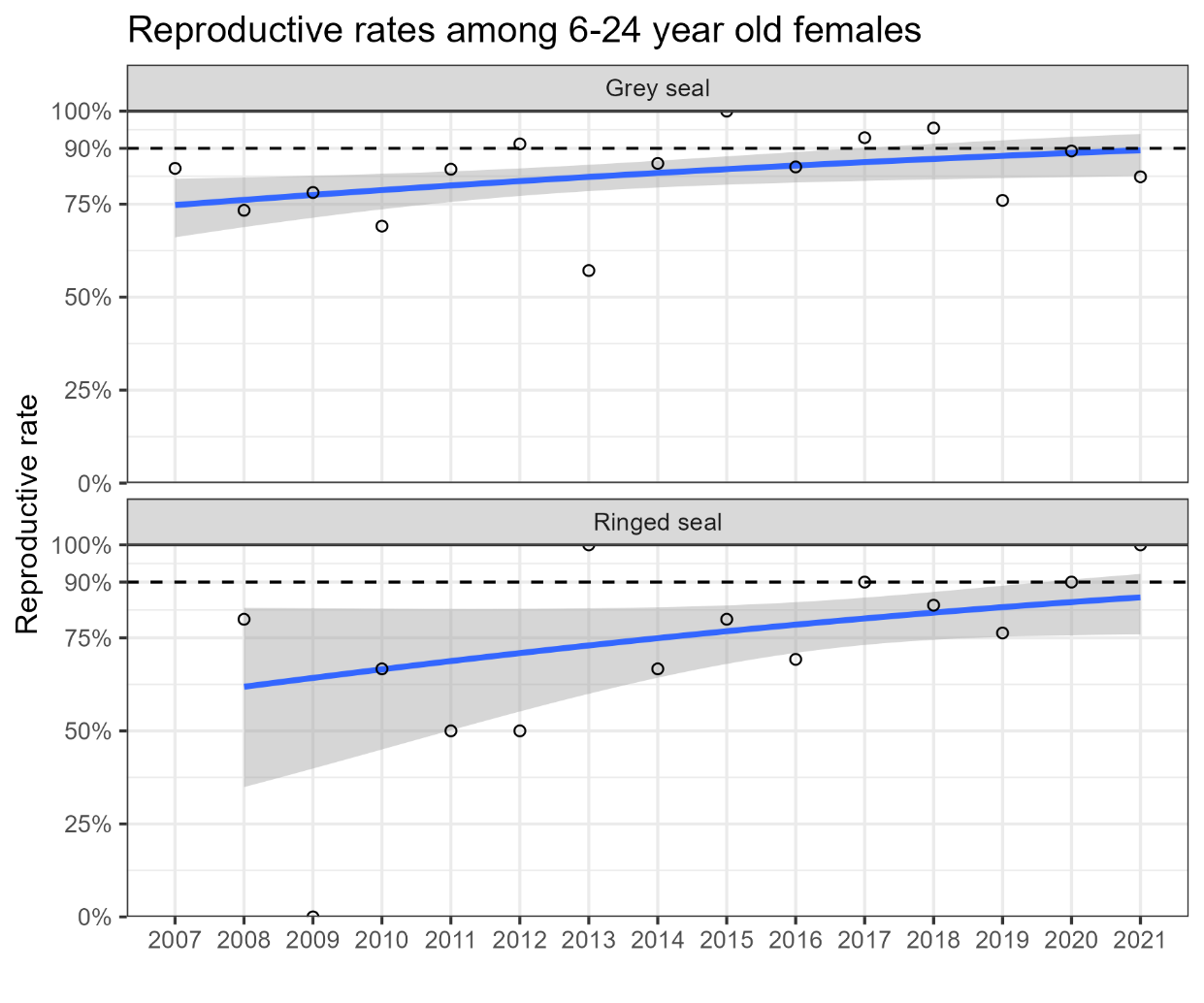 Figure 7. Temporal change in the reproductive status of grey seals and ringed seals. The circles represent the yearly average indicator value (aggregated ratio, see Table 3 and 4) which is used to assess the overall status against the threshold value (90%, dotted line), as shown on the right vertical axis. The blue line is the logistic regression trend line with a 95% point-wise confidence region shaded in grey.
Figure 7. Temporal change in the reproductive status of grey seals and ringed seals. The circles represent the yearly average indicator value (aggregated ratio, see Table 3 and 4) which is used to assess the overall status against the threshold value (90%, dotted line), as shown on the right vertical axis. The blue line is the logistic regression trend line with a 95% point-wise confidence region shaded in grey.
4.3 Discussion
4.3.1 Grey seals
The result of the current evaluation (87%) is similar to the previous assessment period (2011-2016) where pregnancy rate was 83% (HELCOM 2018). During these time periods, the population has increased but not at optimal rates (see population abundance indicator).
The pregnancy rate for females over six years old has been reported to be 87.5% in a large sample of the Northwest Atlantic population (Hamill & Gosselin 1995). In Norwegian, British and Icelandic grey seal populations, the pregnancy rate has varied within approximately 80%-90% (Wiig 1990, Boyd 1999, Hauksson 2007). Thus, the pregnancy rate in Baltic grey seals seems to be within normal ranges and it is proposed that the current threshold value should be reviewed and revised accordingly towards future evaluations.
The method for assessing postpartum signs rate is recommended to be based on placental scars. As placental scars fade with time, it is crucial to investigate the appropriate season for the evaluation. The current evaluation is based on females collected between March-June.
The last case of uterine obstruction in grey seals investigated in Sweden was found in 1993 (Bäcklin et al. 2011). In 2009, one unilateral occlusion was seen in a 13-year old female grey seal in Finland. These seems to be the last observed uterine occlusions in Baltic grey seals.
4.3.2 Ringed seals
The result of the current evaluation (82%) is somewhat higher than during the previous assessment period where the pregnancy rate was found to be 71% for the assessment period 2011-2015 (HELCOM 2018).
As shown in the grey seal, the postpartum signs rate was higher when relying on presence of placental scars rather than involving presence of a corpus albicans. Therefore, the method for assessing postpartum signs rate is recommended to be based on placental scars. As placental scars fade with time, it is important to define the appropriate season for the evaluation. The current evaluation is based on females collected between March-June.
Many studies on ringed seals report only ovulation rates, which shows considerable variations. Although ovulation is not an indicator of pregnancy in seals, it is a prerequisite for fertilization and can therefore reflect the theoretical maximum fertilization rates. Ovulation rates as low as approximately 50% has been reported in relation to episodes of stress in the population (Ferguson et al. 2017).
Some individual ringed seals sampled are still suffering from uterine occlusions. In the 2000s, about 20% of examined Baltic ringed seals still suffered from uterine obstructions, which likely explain the 68% pregnancy rate in ringed seals in 2001-2009 (Helle et al. 2005; Kunnasranta 2010). From the year 2000 up until 2016, data from Finland and Sweden showed that 8.1% of 62 females had occlusions (HELCOM 2018). During the current assessment period (2016-2021), only two ringed seals have been found to have occlusions, indicating a further decline in occlusion frequency. Both these females were >25 years old.
4.3.3. Harbour seals
The number of harbour seals collected from the HELCOM area was insufficient for an evaluation against the threshold of 90% in females over 5 years of age. To be able to make evaluations for the Southwestern Baltic, the Limfjord and Kattegat, a targeted collection strategy needs to be implemented and age determinations must be conducted. The Kalmarsund population is protected and can only be monitored by collecting bycaught and stranded animals.
Gestation rate (presence of a foetus) was 89% (n=80) in harbour seals females 5-24 years of age from the entire Swedish coast (majority of samples from Skagerrak) during the period 2016-2021. This seems to be within normal ranges, as mean pregnancy rate in 3-36 year old harbour seals from Skagerrak-Kattegat was 92% (in 1988). In comparison, mean pregnancy rate in American harbour seals more than 7-8 years of age has been found to be 94% (Boulva & McLaren 1979) and 97% (Bigg 1969), but for 5-year-olds and 6-year-olds the pregnancy rate was lower (55% and 79% respectively, Boulva & McLaren 1979). In a study on Norwegian harbour seals, the pregnancy rate showed a similar pattern with 50% in 6-year-olds and 90% in seals eight years and older (Bjørge 1992). This indicates that the age interval set for the harbour seal populations in the HELCOM area must be investigated and possibly revised. In addition, an upper age limit for inclusion in the evaluation should be investigated.
Monitoring data cover the entire assessment period for grey seals, thus the temporal coverage is sufficient.
For the ringed seal, data was very sparse from 2021, thus the confidence has been set to intermediate for the temporal coverage. Data was sufficient for the ringed seal in the Bothnian Bay, but due to lack of data, the southern management unit could not be assessed; hence the spatial representability was set to intermediate.
The monitoring underlying the reported data were only partly conducted according to the Monitoring guidelines, therefore the confidence in methodology was set to intermediate.
Table 5. Brief summary of pressures and activities with relevance to the indicator.
| | General | MSFD Annex III, Table 2a |
| Strong link | Contamination by hazardous substance
Fisheries and food availability and quality Ecosystem changes (food web, introduction of pathogens and non-indigenous species) Noise pollution Diseases |
Theme: Biological
Theme: Substances, litter and energy Input of other substances (e.g. synthetic substances, non-synthetic substances, radionuclides) |
| Weak link | Hunting | Theme: Substances, litter and energy
Input of anthropogenic sound (impulsive, continuous) |
Changes in the food web related to climate change has the potential to affect the reproduction of all three Baltic seal species strongly. The prey and prey quality have been shown to affect body condition of Baltic seals (Kauhala et al. 2019) and factors affecting body condition will in turn affect reproduction. It is likely that the delayed implantation of seals serves the purpose of ensuring favorable conditions for being pregnant, hence females unable to gain weight after the previous reproductive period will not have a successful implantation and will lose their embryo.
Climate change will limit the availability of ice, which in turn will affect ringed seal reproductive success (Meier et al. 2004, Sundqvist et al. 2012). The ringed seal has a relatively long lactation period. Early ice break-ups may cause the pup to enter the water earlier or more often, which affects their thermoregulation due to the lanugo fur. The pups may be exposed to harsh weather conditions if there is not enough snow and ice for lairs, which poses a risk for hypothermia and a higher mortality (Stirling & Smith, 2004). A shortened period of ice has been observed to increase the number of pups with the lanugo fur still present late in the season and lower growth rates (Harwood et al. 2000, Smith & Harwood 2001).
Less ice coverage may lead to behavioral changes in the populations of ice breeding seals (in the Baltic, ringed and grey seals) that may have consequences. For example, the available territories for the females during the pupping season may decrease, affecting the available food resources and subsequent fertility. An extremely early ice break-up in the Hudson Bay (Canada) in 2010 was related to high levels of cortisol and low ovulation frequency (Ferguson et al. 2017). At the same time, an increased number of sick seals were observed. Similarly, an outbreak of morbillivirus in Caspian seals (Phoca caspica) was preceded by an unusually early ice-break up, which was speculated to have facilitated larger gatherings of seals and/or an increased amount of pups in bad condition and susceptible for infection (Kuiken et al. 2006).
While the grey seal reproductive rate cannot be said to be below what appears to be normal reproductive rates as reported by the scientific literature, it fails to reach the set threshold of 90%.
The ringed seal still suffers from uterine occlusions and although the reproductive rate trend is slowly increasing, the pregnancy rate does not reach the tentative threshold of 90%.
Initiatives across countries to ensure harbour seal collection, necropsy with analysis of reproductive state and age determinations is needed in order to ensure future evaluations.
Although all countries could contribute with data, there were no data reported from several contracting parties/institutions. Lack of age determinations is a limiting factor for using the data that is reported.
8.1 Future work or improvements needed
The threshold of 90% is set based on literature findings across the species (as presented in this report). However, there could be species differences and appropriate age ranges must be compared and applied. In addition, literature reporting reproductive rates based on ovarian CA (or CL) only, is probably not relevant for this indicator. Thus, a revision of the threshold values is recommended towards future evaluations.
9.1 Scale of assessment
This core indicator evaluates the reproductive status of seals using HELCOM assessment unit scale 2 (division of the Baltic Sea into 17 sub-basins). The assessment units are defined in the HELCOM Monitoring and Assessment Strategy Annex 4.
Existing management plans for seals operate according to management units that are based on the distribution of seal populations. The management units typically encompass a handful of HELCOM scale 2 assessment units. Evaluations are therefore done by grouping HELCOM assessment units to align with the management units defined for each seal population.
- The Baltic grey seal (excluding Kattegat) is a single management unit, although genetic data show spatial structuring (Fietz et al. 2013). Behavioural data also suggest some large scale structuring. However, grey seals show extensive migration patterns.
- The Baltic Ringed seal is distributed in the Gulf of Bothnia on the one hand and Southwestern Archipelago Sea, Gulf of Finland and Gulf of Riga on the other, and is represented by two different management units. This sub-division is justified by ecological data that indicate separate dynamics of these stocks (see HELCOM 2018).
- Harbour seals in the Kalmarsund, Sweden constitute a separate management unit and is the genetically most divergent of all harbour seal populations in Europe (Goodman 1998). It was founded about 8,000 years ago and was close to extinction in the 1970s as a consequence of intensive hunting, and possibly also impaired reproduction (Härkönen et al. 2005). The genetic diversity is substantially reduced compared with other harbour seal populations.
- Harbour seals in the southwestern Baltic (Danish Straits, Danish, German and the Öresund region including Skåne county in Sweden and Kattegat) should be managed separately, as this stock is genetically distinct from adjacent populations of harbour seals (Olsen et al. 2014).
- Harbour seals in Kattegat and the Limfjord are genetically distinct from adjacent populations and each other (Olsen et al. 2014), but they are treated as one management unit.
9.2 Methodology applied
This core indicator assesses the reproductive status of seals in the Baltic Sea. The gestation rate is the proportion of sexually mature females with a macroscopically visible embryo/fetus during the period after implantation up until parturition. Sexually mature females can be distinguished by the occurrence of ovulation, where a corpus luteum (CL) subsequently has formed in the ovary. Female seals are sexually mature around the ages 3-5 but are not expected to carry a pup each year. In addition, older females have a declining fertility (Kauhala et al. 2014), therefore the inclusion criteria is 6-24 years old, collected in August-February. The estimated age-specific pregnancy rate increase steeply from the age of four to six (Hamill & Gosselin 1995). Monitoring year is defined as year of expected birth (in February-March for grey seal and ringed seal).
Signs of a previous pregnancy include a placental scar in the uterine horn and a corpus albicans (CA, the remaining structure from a degenerated CL) in the ovary. The presence of a CA in the postpartum period indicates that the female was pregnant in the previous reproductive cycle or that she had an infertile oestrus cycle in the current reproductive cycle (Boyd 1982, 1984). Therefore, the presence of a placental scar should also be used for determining a pregnancy retrospectively, as the presence of a CA only may overestimate the pregnancy rate. Females without an investigation of a placental scar in the uterus were therefore excluded from the evaluation.
As placental scars fade with time, only females collected during the period after parturition up until the expected implantation period of the next pregnancy (for grey seal and ringed seal in March-June) are used for the indicator. In order to compare pregnancy rates over reproductive seasons rather than calendar year, the inclusion age criteria for postpartum signs evaluation is one year older than for investigation of a visible foetus (i.e. 7-25 years old).
Seals in each assessment unit are evaluated against the set threshold values. Samples from opportunistically collected, hunted, by-caught and seals found dead can be used in the analysis. The indicator is based on the investigated presence/absence of a foetus or a placental scar in female seals collected in the prescribed age and seasonal ranges, from August 2015 to June 2021. An unstratified rate is calculated as the total number of presences (regardless of method) divided by the total number of recorded statuses. This value is then assessed against the threshold value of 90%, whence GES is declared if equal or above the threshold and non-GES if below. Rates are also presented stratified by reproductive year and/or method. The evaluation differs from the one taken in HOLAS II, where instead the average of stratified (by reproductive year and method) rates were assessed against the threshold. This change was made to guard against small sample strata receiving too much weight in the evaluation and motivated by an expected small variation in stratified population rates. When sample size is constant over strata, the two approaches are equivalent.
9.3 Monitoring and reporting requirements
Current monitoring is carried out on a national basis, but initiatives of coordinating methodology have been taken by the Health team of the HELCOM Marine mammal expert group. There are natural differences in sources for the collected animals, e.g. in Finland and Sweden hunted seals are predominantly investigated while hunting is not done in the southern Baltic, where collection of stranded carcasses is most commonly investigated. Hunting is not motivated by environmental monitoring but is decided upon by national authorities for other reasons. Age determination by tooth cementum analysis is crucial for inclusion of data for the evaluation in its current form. When monitoring it is important to also record necropsy data and to collect tissue samples for further investigations to find links to the trends in reproductive rate and the causes behind its variation, such as disease, food availability or hazardous substances.
HELCOM monitoring guidelines for reproductive status of seals were updated and accepted on EG HELCOM MAMA meeting in 2021.
The data and resulting data products (e.g. tables, figures and maps) available on the indicator web page can be used freely given that it is used appropriately and the source is cited.
Result: Reproductive status of seals
Data: Reproductive status of seals
The data collected and used in the indicator are based on national databases. The health team of the HELCOM marine mammal expert group is given the responsibility to compile, store current national data, and investigate future arrangements for establishing a HELCOM database.
This version of the HELCOM core indicator report was published in April 2023:
The current version of this indicator (including as a PDF) can be found on the HELCOM indicator web page.
Earlier versions of the core indicator report are available:
Reproductive status of seals HELCOM core indicator 2018 (pdf)
Core indicator report – web-based version December 2015 (pdf)
Extended core indicator report – outcome of CORESET II project (pdf)
Population growth rate, abundance and distribution of marine mammals 2013 (pdf)
Andersen, M., Kovacs, K. M., & Lydersen, C. (2021). Stable ringed seal (Pusa hispida) demography despite significant habitat change in Svalbard, Norway. Polar Research, 40.
Bäcklin, B., Eriksson, L., Olovsson, M. (2003) Histology of uterine leiomyoma and occurrence in relation to reproductive activity in the Baltic grey seal (Halichoerus grypus). Veterinary Pathology 40: 175–180.
Bäcklin, B.-M., Moraeus, C., Roos, A., Eklöf, E., Lind, Y. (2011) Health and age and sex distributions of Baltic grey seals (Halichoerus grypus) collected from bycatch and hunt in the Gulf of Bothnia. ICES Journal of Marine Science 68: 183–188.
Bergman, A., Olsson, M. (1985) Pathology of Baltic grey seal and ringed seal females with special reference to adrenocortical hyperplasia: Is environmental pollution the cause of a widely distributed disease syndrome. Finnish Game Res 44: 47-62.
Bergman, A. (1999) Health condition of the Baltic grey seal (Halichoerus grypus) during two decades. Apmis 107(1‐6): 270-282.
Bernt, K.E., Hammill, M.O., LeBoeuf, M., Kovacs, K.M. (1999) Levels and patterns of PCBs and OC pesticides in harbour and grey seals from the St Lawrence Estuary, Canada. Sci. Total Environ. 243/244: 243-262.
Bigg, M.A. (1969) The harbour seal in British Columbia. Fisheries Research Board of Canada. Bulletin no. 172.
Bignert, A. et al. (1998) Temporal trends of organochlorines in Northern Europe, 1967-1995. Relation to global fractionation, leakage from sediments and international measures. Environ. Poll. 99: 177-198.
Bjørge, A. (1992) The reproductive biology of the harbour seal, Phoca vitulina L., in Norwegian waters.” Sarsia 77.1, 47-51.
Boulva, J., McLaren, I. A. (1979) Biology of the harbor seal, Phoca vitulina, in eastern Canada. Fisheries Research Board of Canada.
Boyd, I.L. 1982. The use of corpora albicantia for determining pregnancy rates in seals with special reference to grey seals (Halichoerus gryphus) ICES report N:14, Retrived 2020-10-17 https://www.ices.dk/sites/pub/CM%20Doccuments/1982/N/1982_N14.pdf
Boyd, I. L. (1984). Development and regression of the corpus luteum in grey seal (Halichoerus grypus) ovaries and its use in determining fertility rates. Canadian Journal of Zoology, 62(6), 1095-1100.
Boyd, I.L., Lockyer C., and Marsh H.D. (1999) Reproduction in marine mammals. In JE. Reynolds III, & JR. Twiss Jr (Eds.), Biology of Marine Mammals pp. 218-286
Bredhult, C., Bäcklin, B.-M., Bignert, A., Olovsson, M. (2008) Study of the relation between the incidence of uterine leiomyomas and the concentrations of PCB and DDT in Baltic gray seals. Reproductive Toxicology 25: 247–255.
European Commission (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora (Habitats Directive). Off. J. Eur. Union 206: 7–50.
European Commission (2000) Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy Off. J. Eur. Union 327: 1-73
European Commission (2008) Directive 2008/56/EC of the European Parliament and the Council establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union L 164: 19-40.
European Commission (2017) Commission Directive amending Directive 2008/56/EC of the European Parliament and of the Council as regards the indicative lists of elements to be taken into account for the preparation of marine strategies.
Ferguson, S.H., Young, B.G., Yurkowski, D.J., Anderson, R., Willing, C., Nielsen, O., 2017. Demographic, ecological, and physiological responses of ringed seals to an abrupt decline in sea ice availability. PeerJ 5, e2957.
Fietz, K., Graves, J.A., Olsen, M.T. (2013) Control Control Control: A Reassessment and Comparison of GenBank and Chromatogram mtDNA Sequence Variation in Baltic Grey Seals (Halichoerus grypus). PLoS ONE 8(8): e72853. doi:10.1371/journal.pone.0072853.
Goodman, S.J. (1998) Patterns of extensive genetic differentiation and variation among European harbor seals (Phoca vitulina vitulina) revealed using microsatellite DNA polymorphisms. Molecular Biology and Evolution 15(2): 104-118.
Hamill M.O., Gosselin J.F. (1995) Reproductive rates, age of maturity and age at first birth in Northwest Atlantic grey seals (Halichoerus grypus). Ca J. Fish. Aquat. Sci. 52: 2757-2761.
Harding, K.C., Härkönen, T., Helander, B., Karlsson, O. (2007) Status of Baltic grey seals: Population assessment and risk analysis. NAMMCO Scientific Publications 6: 33-56.
Harwood, L.A., Smith, T.G., Melling, H., 2000. Variation in Reproduction and Body Condition of the Ringed Seal (Phoca hispida) in Western Prince Albert Sound, NT, Canada, as Assessed through a Harvest-Based Sampling Program. Arctic 53, 422–431.
Hauksson, E. (2007). Growth and reproduction in the Icelandic grey seal. NAMMCO Scientific Publications, 6, 153-162.
Heide-Jørgensen, M.-P., Härkönen, T. (1992) Epizootiology of seal disease. J. Appl. Ecol. 29: 99-107
HELCOM (2018) Reproductive status of marine mammals. HELCOM core indicator report. Online. <http://www.helcom.fi/baltic-sea-trends/indicators/reproductive-status-of-seals/>.
HELCOM (2021): HELCOM Baltic Sea Action Plan– 2021 update
Helle, E. (1979) Structure and number of seal populations in the northern Baltic Sea: a study based on Finnish bounty statistics, 1956-1975. Aquilo Ser. Zool. 19: 65-71.
Helle, E. (1980) Lowered reproductive capacity in female ringed seals (Pusa hispida) in the Bothnian Bay, northern Baltic Sea, with special reference to uterine occlusions. Annales Zoologica Fennici 17: 147-158.
Helle, E., Nyman, M., Stenman, O. (2005) Reproductive capacity of grey and ringed seal females in Finland. International conference on Baltic seals, 15–18 February 2005. Helsinki, Finland.
Kauhala, K., Ahola, M. P., & Kunnasranta, M. (2014) Decline in the pregnancy rate of Baltic grey seal females during the 2000s. In Annales Zoologici Fennici (Vol. 51, No. 3, pp. 313-324). Finnish Zoological and Botanical Publishing Board.
Kauhala, K., Bergenius, M., Isomursu, M., Raitaniemi, J., (2019). Reproductive rate and nutritional status of Baltic ringed seals. Mammal Res. 64, 109–120.
Kjellqvist, S.A., Haug, T., Øritsland, T. (1995) Trends in age-composition, growth and reproductive parameters of Barents Sea harp seals, Phoca groenlandica. ICES Journal of Marine Science 52: 197–208.
Kuiken, T., Kennedy, S., Barrett, T., Van de Bildt, M.W.G., Borgsteede, F.H., Brew, S.D., Codd, G.A., Duck, C., Deaville, R., Eybatov, T., Forsyth, M.A., Foster, G., Jepson, P.D., Kydyrmanov, A., Mitrofanov, I., Ward, C.J., Wilson, S., Osterhaus, A.D.M.E., (2006). The 2000 Canine Distemper Epidemic in Caspian Seals (Phoca caspica): Pathology and Analysis of Contributory Factors. Vet. Pathol. 43, 321–338.
Kunnasranta, M. (2010) Merihylkeet vuonna 2010. Riistajakalatalous. Selvityksiä 21/2010: 21–22.
Meier, H.E.M., Döscher, R., Halkka, A. (2004) Simulated distributions of Baltic Sea-ice in the warming climate and consequences for the winter habitat of the Baltic Ringed Seal. Ambio 33: 249–256.
Olsen, M.T., Wesley Andersen, L., Dietz, R., Teilmann, J., Harkonen, T., Siegismund, H.R. (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology 23: 815-831.
Reijnders, P.J.H. (1986) Reproductive failure in common seals feeding on fish from polluted coastal waters. Nature 324: 456-457
Safe, S. (1984) Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanisms of action. CRC Crit. Rev. Toxicol. 13: 319-395.
Siebert U., Müller S., Gilles A., Sundermeyer J., Narberhaus U.I. Species profiles marine mammals. In: Narberhaus I., Krause J., Bernitt U., editors. Threatened Biodiversity in the German North and Baltic Seas – Sensitivities towards Human Activities and the Effects of Climate Change. vol. 116. 2012. pp. 487–542. (Naturschutz und Biologische Vielfalt).
Smith, T. G. (1970) Population dynamics of the ringed seal in the Canadian Eastern Arctic. Thesis in Marine Sciences. McGill University, Montreal, Canada
Smith, T.G., Harwood, L.A., (2001) Observations of neonate ringed seals, Phoca hispida, after early break-up of the sea ice in Prince Albert Sound, Northwest Territories, Canada, spring 1998. Polar Biol. 24, 215–219.
Stirling, I., Smith, T.G., (2004) Implications of Warm Temperatures and an Unusual Rain Event for the Survival of Ringed Seals on the Coast of Southeastern Baffin Island. Arctic 57, 59–67.
Sundqvist, L., Harkonen, T., Svensson, C.J., Harding, K.C. (2012) Linking climate trends to population dynamics in the Baltic ringed seal – Impacts of historical and future winter temperatures. Ambio. 41.8
Wiig, Ø. (1991) Demographic parameters for Norwegian grey seals, Halichoerus grypus. Fauna Norv. Ser A.12:25-28.
Harding, K.C., Härkönen, T.J. (1999) Development in the Baltic grey seal (Halichoerus grypus) and ringed seal (Phoca hispida) populations during the 20th century. Ambio 28: 619-627.
Harding, K.C., Fujiwara, M., Härkönen, T., Axberg, Y. (2005) Mass dependent energetics and survival in harbour seal pups. Functional Ecology 19: 129-135.
Härkönen, T., Heide-Jørgensen, M.-P. (1990) Density and distribution of the ringed seal in the Bothnian Bay. Holarctic Ecology 13 (2): 122-129.
Härkönen, T., Harding, K.C. (2001) Spatial structure of harbour seal populations and the implications thereof. Can. J. Zool. 79: 2115-2127.
Härkönen, T., Harding, K.C., Goodman, S., Johannesson, K. (2005) Colonization history of the Baltic harbor seals: Integrating archaeological, behavioural and genetic data. Marine Mammal Science 21: 695-716.
Jensen, S., Johnels, A.G., Olsson, M., Otterlind, G. (1969) DDT and PCB in marine animals from Sweden. Nature 224: 247-250.
Jepson, P. D., Deaville, R., Barber, J. L., Aguilar, À., Borrell, A., Murphy, S., Barry, J., Brownlow, A., Barnett, J., Berrow, S., Cunningham, A. A., Davison, N. J., Doeschate, M., Esteban, R., Penrose, R., Perkins, M. W., Smith, B., Stephanis, R. De, Tregenza, N., Verborgh, P., Fernández, A. and Law, R. J. 2016. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Nature Scientific Reports, 6:18573.
Olsson, M. (1977) Mercury, DDT and PCB in aquatic test organisms. Baseline and monitoring studies, field studies on biomagnification, metabolism and effects of some bioaccumulating substances harmful to the Swedish environment. Report from the National Swedish Environment Protection Board 1977. SNV PM 900 139pp.
Oksanen, S. M., Niemi, M. Ahola, M. P. & Kunnasranta, M. 2015. Identifying foraging habitats of Baltic ringed seals using movement data. Movement Ecology 3:33.
Jüssi, M., Härkönen, T., Jüssi, I. Helle, E. (2008) Decreasing ice coverage will reduce the reproductive success of Baltic grey seal (Halichoerus grypus) females. Ambio 37: 80–85.
Kauhala, K., Ahola, M. & Kunnasranta, M. 2014. Decline in the pregnancy rate of Baltic grey seal females during the 2000s, estimated with different methods. – Annales Zoologici Fennici 51: 313-324.
Teilmann, J., Riget, F., Harkonen, T. (2010) Optimising survey design in Scandinavian harbour seals: Population trend as an ecological quality element. ICES Journal of Marine Science 67: 952–958.
Vanhatalo, J., Vetemaa, M., Herrero, A., Aho, T., Tiilikainen, R. (2014) By-Catch of Grey Seals (Halichoerus grypus) in Baltic Fisheries—A Bayesian Analysis of Interview Survey. PLoS ONE 9(11): e113836.
Zohari, S., Neimanis, A., Härkönen, T., Moraeus, C., Valarcher, J.F. (2014) Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill. 19(46): pii=20967.