 Soft-bottom macrofauna
Soft-bottom macrofauna
2 Relevance of the indicator
Soft-sediment macrofaunal species in the Baltic Sea include animals such as bivalves, polychaetes and crustaceans. The animals live on the seafloor as well as burrowed into the sediments, thus forming an important link between sediments and the water column. Soft-sediment macrofaunal species act as suspension feeders, predators and decomposers and form an important link in the marine food web by coupling the pelagic and benthic environments as well as constituting an important food source for other animals, such as fish and water birds.
This indicator evaluates the status of the environment using an index developed to show changes in the relative abundance of sensitive and tolerant species, as well as the diversity of the community in soft sediments.
2.1 Ecological relevance
Macrofaunal species live either on top of the sediment or in the sediment as infauna. The macrozoobenthic community influences the marine nutrient turnover by coupling biological and physicochemical cycles of both compartments, known as the benthic-pelagic coupling. Foraging and burrowing activities in sediment influences the oxygenation of the sediment and other biogeochemical processes. In addition to forming a link between the water mass and the sediments, the macrozoobenthic species also form an important link in the marine food web. Many of the macrozoobenthic species are primary consumers that filter particles from the water or graze on and in the sediments, while others are predators and scavengers. Furthermore, many marine top-predators feed on these macrozoobenthic species. Moreover, as the main part of the seafloor is covered by soft sediments, the macrozoobenthic community is a key component to be considered in any evaluation of the status of the environment.
The composition of the macrozoobenthic community varies across environmental gradients and reflects parameters such as salinity, oxygen, food supply, biotic interactions and hydrological conditions. Changes in environmental parameters will result in changes in community composition. In addition to changes due to natural environmental fluctuations, the composition is also affected by anthropogenic pressures. Generally, Baltic soft-bottom macrofauna are characterized by small shallow-dwelling species, owing to low salinity and transient hypoxia. Evolutionarily mature benthic communities composed of deeper-dwelling and/or larger species developed in the southern Baltic, for example: some long-lived bivalves and large polychaetes. In the open sea areas of the northern subbasins, the soft-bottom macrofauna community is dominated by a small number of species, including for example: the amphipods Monoporeia affinis and Pontoporeia femorata, the isopod Saduria entomon, the polychaets Bylgides sarsi and Marenzelleria spp., and the bivalve Limecola balthica. In the open sea areas of the southern subbasins the communities are markedly different with a dominance of clearly marine species, including for example: the bivalves Arctica islandica and Astarte borealis and numerous species of polychaetes. This latitudinal distribution pattern is defined by the gradient of decreasing salinity towards the northeast, which decreases soft-bottom macrofauna diversity, affecting both the structure and function of the communities (Elmgren 1989, Rumohr et al. 1996, Bonsdoff & Pearson 1999, Villnäs & Norkko 2011, Gogina et al. 2016).
In the Baltic proper, the distribution of soft-bottom macrofauna communities is also driven by strong vertical gradients. Generally, more species-rich and abundant communities are found in shallow-water habitats (with higher habitat diversity) compared to the deep-water communities which are dominated by only a few species (Andersin et al. 1978). The Baltic Proper has a more or less permanent halocline at 60-80 m, whereas in the Gulf of Bothnia stratification is weak or absent. The halocline in deeper waters and seasonal pycnoclines in coastal waters restrict vertical water exchange, which may result in oxygen deficiency, a factor that is undoubtedly the most significant threat to the biodiversity of Baltic Sea soft-sediment macrofauna communities. In the open sea, the communities have for several decades been severely affected by oxygen depletion. Current evidence suggests that the spatial and temporal extent of oxygen deficiency has increased over the past decades. Consequently, only the area above the halocline, i.e., not suffering from permanent hypoxia, is evaluated using this indicator.
The soft-bottom macrofaunal species that make up the community have different characteristics and react differently to anthropogenic pressures. Thus, an evaluation of the community composition and abundances of species in the community for a specific area represents a good indicator for evaluating the status of the environment. In the further south regions of the Baltic Sea where conditions are more marine (i.e., higher salinity) the macrozoobenthic community is considered to be a good indicator due to the fact that some species are relatively stationary and long-lived (years to decades). Monitoring such species enables the integration of environmental information and reduces fluctuations in the dataset once natural variability has been taken appropriately into account, and increases certainty should few samples be required to represent a larger area or time period.
The evaluation results show good status for most basins, even in eutrophic or otherwise impacted areas. As the indicator concept is based on sensitive species and diversity, the impacts of the main pressures affecting the benthic fauna are expected to be reflected in the evaluation results. Further development of the indicator and its evaluation thresholds may be needed to achieve better alignment between the evaluation of pressures and their impacts on benthic habitats and benthic fauna.
2.2 Policy relevance
This core indicator has mainly been developed with the aim to evaluate the HELCOM ecological objectives under the biodiversity segment goal of. ‘Baltic Sea ecosystem is healthy and resilient ‘ (Baltic Sea Action Plan 2021). These include: ‘Viable populations of all native species’, ‘Natural distribution, occurrence and quality of habitats and associated communities’, and to an extent is also relevant when addressing ‘Functional, healthy and resilient food webs’. Some information of relevance can be gained also when addressing ‘Natural distribution and occurrence of plants and animals’ and ‘Natural oxygen levels’ under the eutrophication segment.
Under the EU Marine Strategy Framework Directive, the indicator describes the state of benthic habitats linking to descriptor 6: Sea-floor integrity (Criterion D6C5), descriptor 5: Eutrophication (Criterion D5C8) and also contributes to descriptor 4: Food webs (Criterion D4C1). The indicator addresses the environmental state of macrofaunal species of soft-bottom habitats, but it is not calibrated to translate directly to the Broad Habitat Types (BHTS) assessed under the MSFD.
The indicator on State of the soft-bottom macrofauna community addresses the Baltic Sea Action Plan (BSAP 2021) vision of a healthy and resilient Baltic Sea ecosystem and a more detailed overview of policy relevant elements is provided on Table 1.
Table 1. Summary of policy relevance of this indicator.
| Baltic Sea Action Plan (BSAP) | Marine Strategy Framework Directive (MSFD) | |
| Fundamental link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
|
Descriptor 6 Benthic habitats – Benthic broad habitat types.
Note: this indicator is not considered as a comprehensive solution to D6C5 but as a component contributing to the assessment of D6C5. |
| Complementary link | Segment: Biodiversity
Goal: “Baltic Sea ecosystem is healthy and resilient”
Segment: Eutrophication Goal: “Baltic Sea unaffected by eutrophication”
Segment: Hazardous substances and Litter Goal: “Baltic Sea unaffected by hazardous substances and litter”
|
Descriptor 5 Human-induced eutrophication is minimised, especially adverse effects thereof, such as losses in biodiversity, ecosystem degradation, harmful algae blooms and oxygen deficiency in bottom waters – Macrofaunal communities of benthic habitats.
Descriptor 4 Ecosystems, including food webs – Trophic guilds of an ecosystem.
|
| Other relevant legislation: |
|
|
2.3 Relevance for other assessments
The biodiversity status is assessed using several core indicators. Each indicator focuses on one important aspect of this complex issue. In addition to providing an indicator-based evaluation, this indicator will also contribute to the integrated biodiversity and eutrophication assessments in the respective Thematic Assessments under the Stathe of the Baltic Sea report (HOLAS 3).
3 Threshold values
Thresholds values have been set using two different approaches, dependent on which method for species sensitivity values was used (sub-region specific). The threshold value concept is a defined value that should be achieved in order to indicate good status (Figure 2).
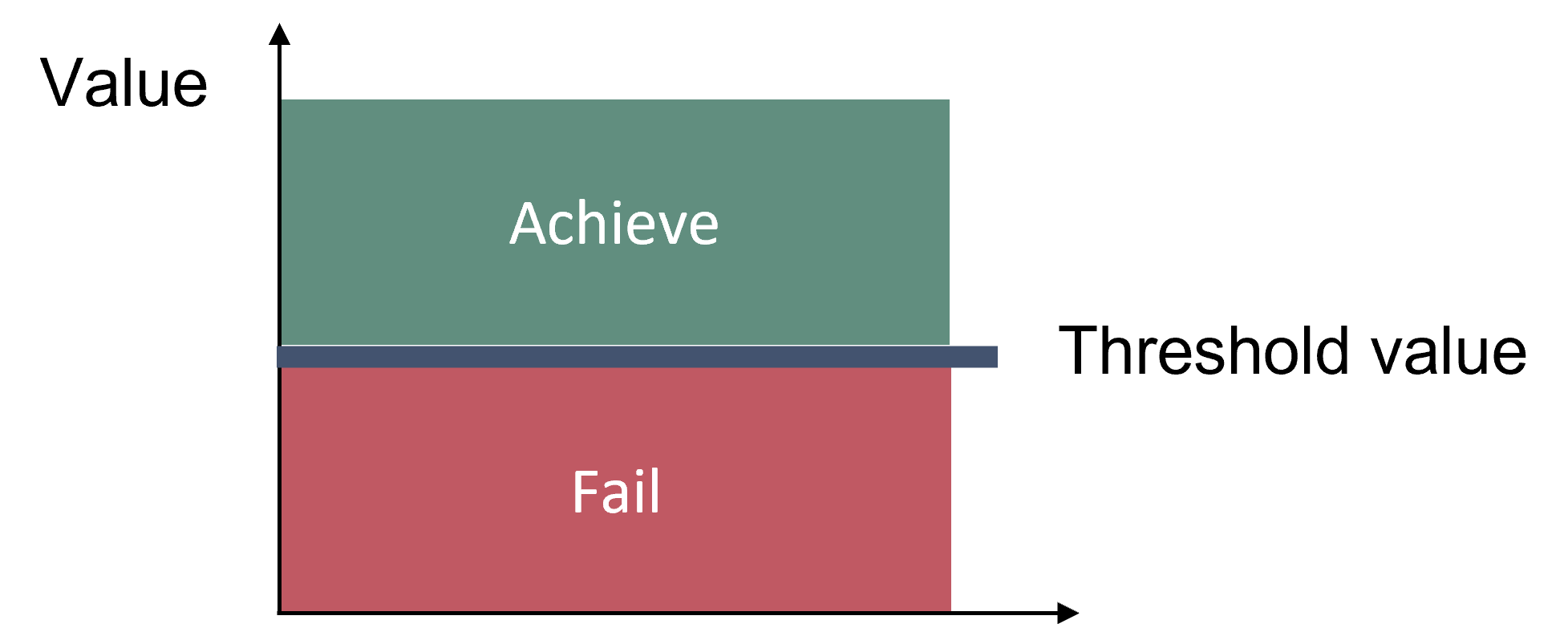
Figure 2. Schematic figure of the threshold value concept applied in the core indicator.
In the Bothnian Bay, The Quark, Bothnian Sea, Åland Sea, Northern Baltic Proper and Western Gotland Basin, where the method follows Leonardsson et al. (2009), the Swedish intercalibrated BQI good-moderate threshold values, developed for outer coastal waters under the EU Water Framework Directive, are considered to also be applicable for the open sea assessment units. The establishment of these threshold values is based on both statistical tests and expert judgment, using data from areas without local disturbance to define high and good status as baselines, as described in Leonardsson et al. (2009).
In Gulf of Finland, Gulf of Riga, Eastern Gotland Basin, Bay of Mecklenburg and Kiel Bay the threshold is defined for each used subset described in Schiele et al. (2016). In this method the described fauna sub-sets that occur in the assessment unit are first identified. Threshold values are then calculated for each subset according to a pragmatic statistical scheme developed by Perus et al. (2007) and later modified during an intercalibration process, as described by Carletti & Heiskanen (2009). In short, this method sets threshold values as 0.6 times the 10th percentile of the top 10 % of all index values within a subset.
Threshold values for all open sea assessment units are shown in Table 2. No threshold value has been agreed for the following open sea assessment units: Kattegat, Great Belt, the Sound, Arkona Basin, Bornholm Basin and Gdansk Basin. The indicator is in principle applicable in these areas, and further work for these assessment units is underway.
Table 2. Threshold values used in the open sea assessment units. In Bothnian Bay, The Quark, Bothnian Sea, Åland Sea, Northern Baltic Proper and Western Gotland Basin one threshold value per unit is given, whereas in Gulf of Finland, Gulf of Riga, Eastern Gotland Basin, Bay of Mecklenburg and Kiel Bay one threshold value per subset, irrespective of assessment unit is given. Thus one assessment unit may have more than one threshold value. Note that threshold values in assessment units where the Schiele et al. (2016) sensitivity value method is used will be 0.5 after normalisation to a common scale (see Assessment protocol).
| Open sea assessment unit |
Evaluated depths | Threshold value | BQI species sensitivity value method | ||||||||
| Subset according to Schiele et al. 2016 | |||||||||||
| 2 | 3 | 4 | 8 | 9 | 11 | 12 | 13 | ||||
| Bothnian Bay | 1.5 | Leonardsson et al. 2009 | |||||||||
| The Quark | 1.5 | ||||||||||
| Bothnian Sea | 4.0 | ||||||||||
| Åland Sea | 4.0 | ||||||||||
| Northern Baltic Proper | <60 m | 4.0 | |||||||||
| Western Gotland Basin | <60 m | 4.0 | |||||||||
| Gulf of Finland | <60 m | 0.93 | 1.07 | Schiele et al. 2016 | |||||||
| Gulf of Riga | 0.93 | 1.59 | 1.07 | ||||||||
| Eastern Gotland Basin | <60 m | 4.0* | 1.81 | 2.11 | 1.07 | ||||||
| Bay of Mecklenburg | 7.22 | 5.44 | 4.52 | ||||||||
| Kiel Bay | 7.22 | 5.44 | 4.52 | ||||||||
| *Using species sensitivity values according to Leonardsson et al. 2009 | |||||||||||
3.1 Setting the threshold value(s)
Thresholds values differ sub-regionally and depend on the specific method and sensitivities used. The Leonardsson et al. (2009) methodology was developed for outer coastal waters under the EU Water Framework Directive and is also applicable for open sea assessment units. Where applied, the establishment of these threshold values is based on both statistical tests and expert judgment, using data from areas without local disturbance to define high and good status as baselines, as described in Leonardsson et al. (2009). Where the Schiele et al. (2016) sub-sets are used, the method is based on a description of the fauna sub-sets that occur in the assessment unit and threshold values are then calculated for each subset according to a pragmatic statistical scheme developed by Perus et al. (2007) and later modified during an intercalibration process (Carletti & Heiskanen, 2009). In short, this method sets threshold values as 0.6 times the 10th percentile of the top 10 % of all index values within a subset.
4 Results and discussion
The results of the indicator evaluation that underly the key message map and information are provided below.
4.1 Status assessment
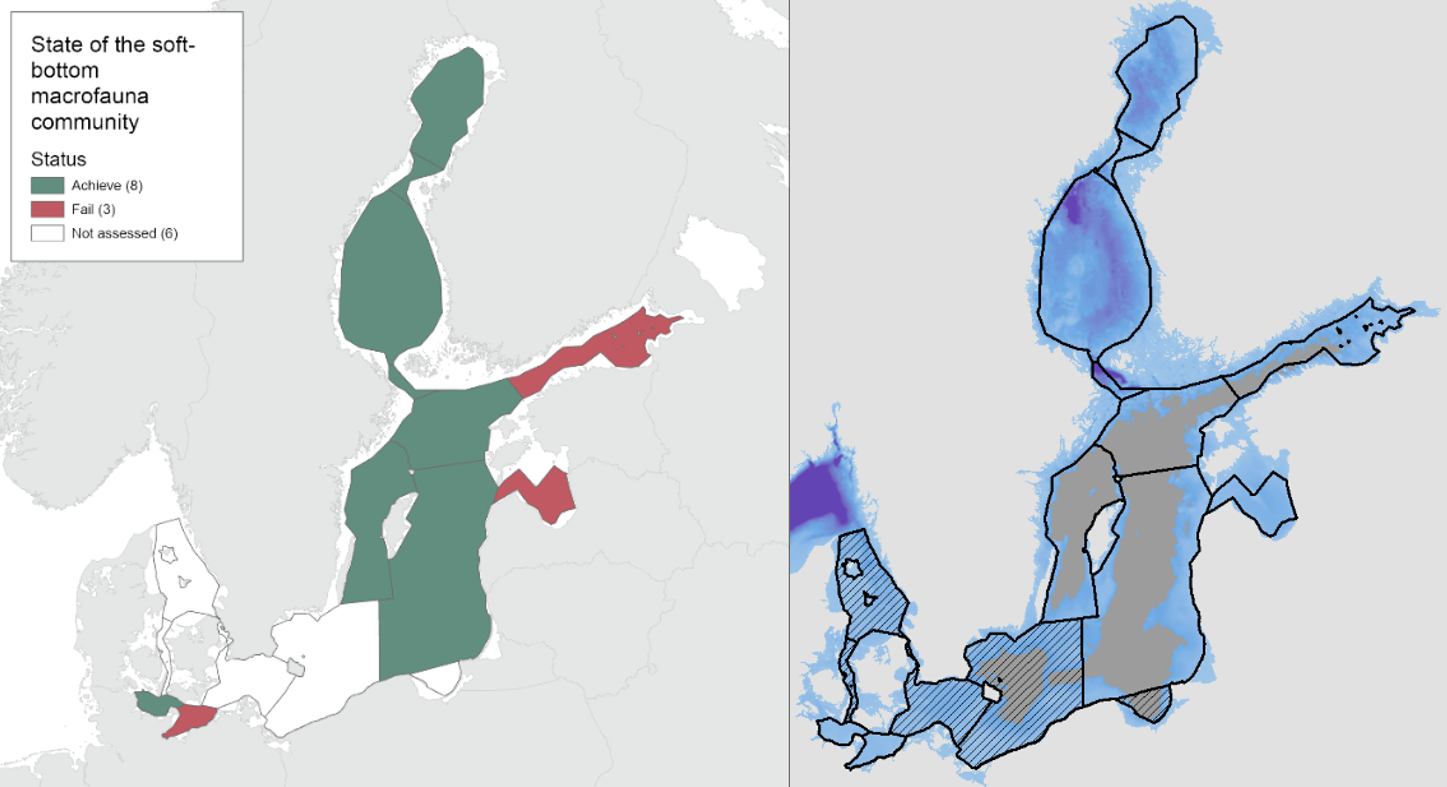
During the 2016-2021 period, the results indicated good status in all the evaluated open sea assessment units apart from the Bay of Mecklenburg, Gulf of Riga and Gulf of Finland (Figure 3).
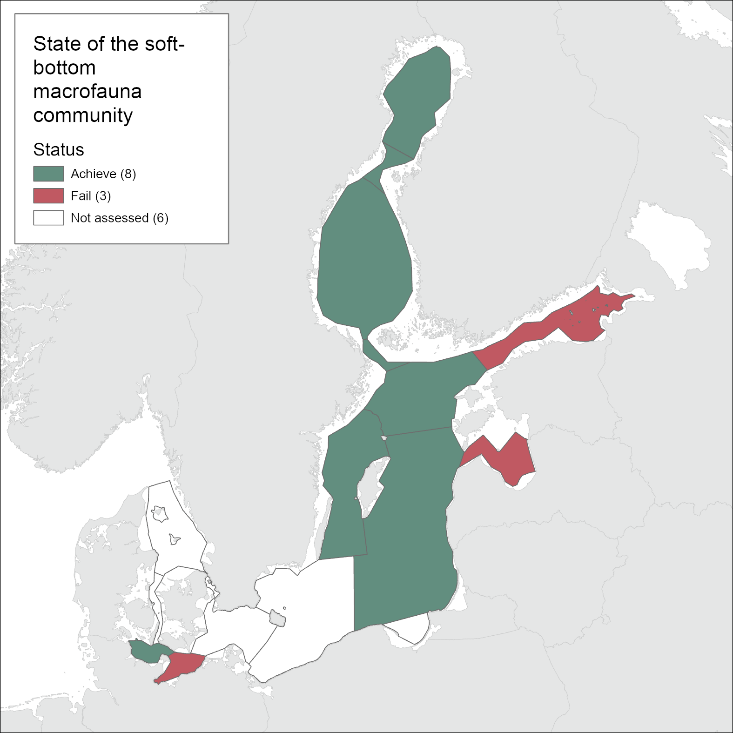
Figure 3. Status evaluation of the sub-basins evaluated in the current assessment period (2016-2021).
It should be noted that the indicator only represents the areas above the permanent halocline (< 60m depth, see Figure 1 for details). This approach is instituted since significant areas within the Northern Baltic Proper, Gulf of Finland, Eastern Gotland Basin and Western Gotland Basin suffer from hypoxia. No reliable threshold value capable of differentiating between the status of areas only affected by natural hypoxia or those hypoxic areas driven by anthropogenic eutrophication was definable. Thus, it is recommended that the oxygen debt indicator is used to evaluate the status below the halocline in these subbasins.
4.2 Trends
Compared to the HOLAS II assessment period 2011-2016, the status classification has worsened in the Gulf of Riga and the Gulf of Finland from achieving the threshold in HOLAS II to below the threshold in the assessment period 2016-2021 (HOLAS 3). In all other evaluated assessment units the status classification remained unchanged with the indicator result achieving the GES threshold values, apart from in the Bay of Mecklenburg where the status remain below the threshold.
4.3 Discussion
The results from which the above described status evaluation is derived are provided below per evaluated sub-basin (Figure 4).
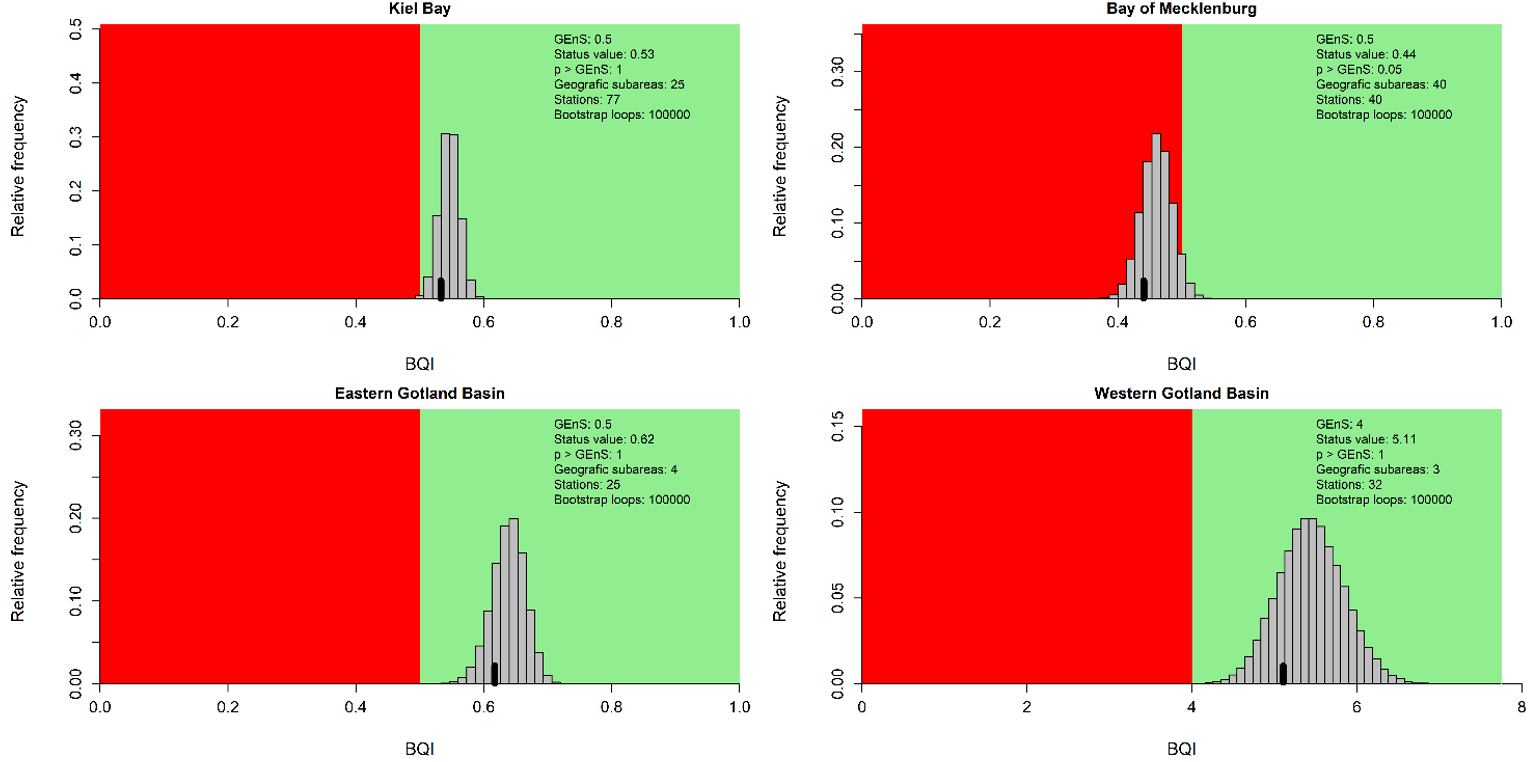
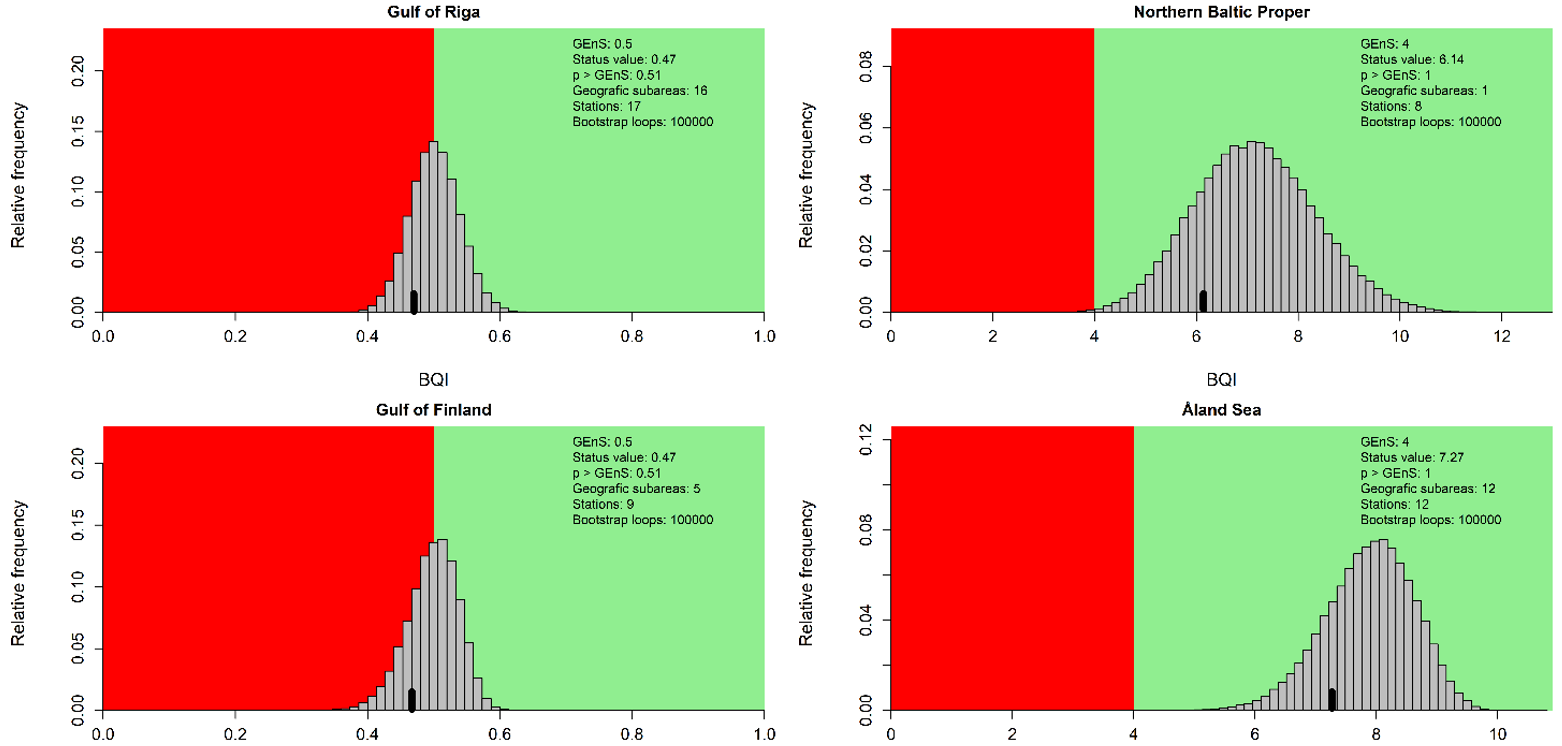
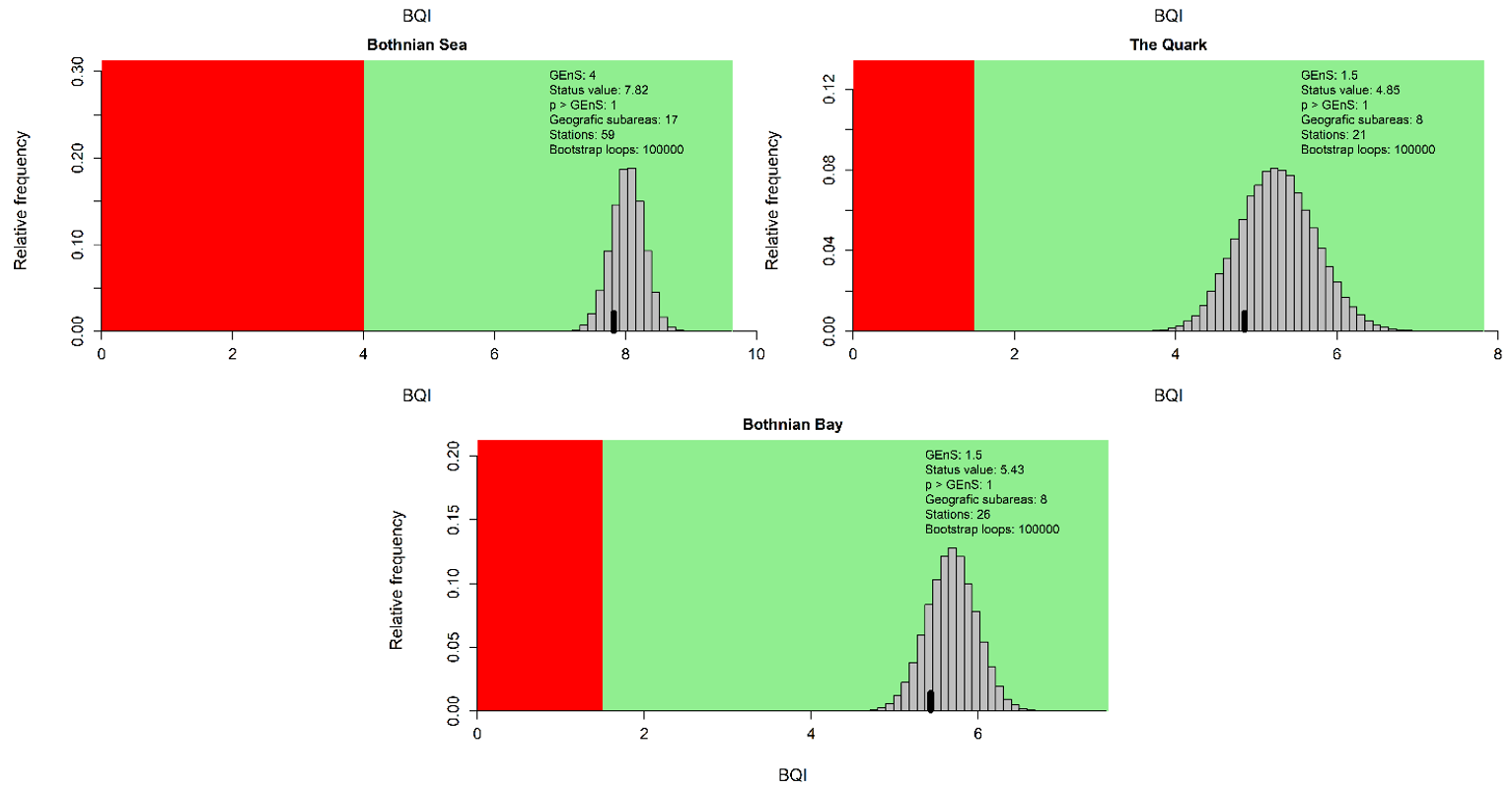
Figure 4. Evaluation results for the subbasin open sea assessment units. The histograms reflect the distribution of the 100 000 bootstrapped mean BQI values and the black bar show the evaluation value (20th percentile) which is compared to the threshold value (see Assessment Protocol for details). The red and green areas correspond to failing and achieving the assessment unit specific threshold value respectively.
The indicator result is relevant for all soft-bottom macrofaunal communities, but it is not calibrated to translate directly to the MSFD BHTS. To address how well the indicator results represent the broad habitat types in the respective assessment units information on broad habitat type was extracted by overlaying the sampling stations on EUSeaMap 2021 (Table 3). Whereas the samples used in the indicator calculations are confirmed as taken on soft-bottoms, i.e., suitable for grab sampling, it has to be noted that the scale of the EUSeaMap is too coarse to reflect the true representativity of the samples. As a comparison to the distribution of samples across the different broad habitat types, the proportional areas of each broad habitat type in the evaluated area of the respective assessment units are presented in Table 4. These issue reflect the need for improved habitat mapping to support better benthic habitat evaluations in the future.
Table 3. Distribution of samples and stations (in brackets) among EUSeaMap 2021 defined broad habitat types in the respective assessment units.
| Broad habitat type EUSeaMap 2021 | Kiel Bay | Bay of Mecklenburg | Eastern Gotland Basin | Western Gotland Basin | Gulf of Riga | Northern Baltic Proper | Gulf of Finland | Åland Sea | Bothnian Sea | The Quark | Bothnian Bay |
| Infralittoral coarse sediment | 39 (5) |
29 (6) |
12 (2) |
1 (1) |
|||||||
| Infralittoral mixed sediment | 6 (1) |
2 (1) |
|||||||||
| Infralittoral mud | 6 (2) |
||||||||||
| Infralittoral sand | 54 (14) |
88 (13) |
36 (2) |
||||||||
| Circalittoral rock and biogenic reef |
8 (1) |
11 (4) |
3 (1) |
17 (1) |
6 (2) |
||||||
| Circalittoral coarse sediment | 20 (6) |
22 (4) |
23 (2) |
21 (3) |
|||||||
| Circalittoral mixed sediment | 42 (5) |
76 (26) |
17 (6) |
12 (4) |
161 (33) |
30 (10) |
39 (9) |
||||
| Circalittoral mud | 10 (4) |
78 (20) |
211 (12) |
1 (1) |
64 (7) |
21 (7) |
3 (1) |
18 (2) |
|||
| Circalittoral mud or Circalittoral sand |
90 (8) |
34 (4) |
3 (1) |
24 (2) |
31 (7) |
90 (14) |
57 (9) |
75 (14) |
|||
| Circalittoral sand | 60 (30) |
40 (6) |
15 (3) |
3 (1) |
3 (1) |
||||||
| Offshore circalittoral coarse sediment |
9 (9) |
||||||||||
| Offshore circalittoral mud | 3 (3) |
||||||||||
| Offshore circalittoral sand | 4 (4) |
||||||||||
| Total | 205 (77) |
235 (42) |
231 (25) |
93 (32) |
268 (15) |
24 (8) |
88 (9) |
60 (12) |
299 (59) |
90 (20) |
135 (26) |
Table 4. Relative distribution (in %) of broad habitat types according to EUSeaMap 2021 in the evaluated area (open sea areas and only areas <60 m deep in Eastern Gotland Basin, Western Gotland Basin, Northern Baltic Proper and the Gulf of Finland), in the respective assessment units.
| Broad habitat type EUSeaMap 2021 | Kiel Bay | Bay of Mecklenburg | Eastern Gotland Basin | Western Gotland Basin | Gulf of Riga | Northern Baltic Proper | Gulf of Finland | Åland Sea | Bothnian Sea | The Quark | Bothnian Bay |
| Infralittoral rock and biogenic reef |
<0.1 | 0.4 | 0.7 | <0.1 | 0.4 | 0.9 | 0.1 | <0.1 | 0.1 | <0.1 | |
| Infralittoral coarse sediment | 9.3 | 4.3 | 4.6 | 2.3 | 0.8 | <0.1 | <0.1 | <0.1 | 0.1 | <0.1 | <0.1 |
| Infralittoral mixed sediment | 11.4 | 8.0 | 5.5 | 9.3 | 0.2 | 3.5 | 0.6 | 0.1 | 0.1 | 0.5 | 0.1 |
| Infralittoral mud | 1.8 | 2.6 | 0.1 | <0.1 | <0.1 | 0.7 | <0.1 | ||||
| Infralittoral mud or Infralittoral sand |
0.4 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |||
| Infralittoral sand | 39.3 | 44.6 | 2.2 | 0.4 | 0.4 | 0.1 | 0.5 | <0.1 | <0.1 | 0.3 | |
| Circalittoral rock and biogenic reef |
1.7 | 7.6 | 0.1 | 8.1 | 3.7 | 8.0 | 0.9 | 0.6 | <0.1 | ||
| Circalittoral coarse sediment | 1.4 | 0.8 | 16.4 | 2.4 | 4.1 | 1.6 | 7.5 | 2.6 | 1.8 | 8.6 | 2.7 |
| Circalittoral mixed sediment | 3.6 | 1.4 | 18.6 | 70.6 | 3.8 | 41.5 | 17.6 | 34.1 | 63.0 | 67.5 | 40.1 |
| Circalittoral mud | 10.7 | 29.0 | 6.0 | 3.5 | 61.1 | 19.5 | 17.1 | 1.6 | 2.0 | 2.9 | 2.4 |
| Circalittoral mud or Circalittoral sand |
14.1 | 1.1 | 17.3 | 19.9 | 25.3 | 53.4 | 31.0 | 15.0 | 33.4 | ||
| Circalittoral sand | 22.1 | 9.3 | 29.9 | 1.7 | 12.2 | 0.3 | 12.1 | 0.9 | 4.8 | 20.9 | |
| Offshore circalittoral rock and biogenic reef |
<0.1 | 0.4 | |||||||||
| Offshore circalittoral coarse sediment |
<0.1 | <0.1 | <0.1 | 1.1 | <0.1 | ||||||
| Offshore circalittoral mixed sediment |
<0.1 | 0.1 | 0.2 | 1.5 | 1.6 | <0.1 | |||||
| Offshore circalittoral mud | 0.1 | <0.1 | 0.1 | 0.8 | 4.4 | ||||||
| Offshore circalittoral mud or Offshore circalittoral sand |
<0.1 | 2.1 | 4.0 | <0.1 | |||||||
| Offshore circalittoral sand | 0.3 | <0.1 | 2.3 | <0.1 | |||||||
| Na | 0.9 |
An overall summary of the current status evaluation and a comparison between the current and previous assessment period is provided in Table 5.
Table 5. Summary of current status evaluation (2016-2021) and comparison with previous assessment period (2011-2016).
| HELCOM Assessment unit name (and ID) | Threshold value achieved/failed – HOLAS II | Threshold value achieved/failed – HOLAS 3 | Distinct trend between current and previous evaluation. | Description of outcomes, if pertinent. |
| Kiel Bay | Achieved | Achieved | No change in classification | |
| Bay of Mecklenburg | Failed | Failed | No change in classification | |
| Eastern Gotland Basin | Achieved | Achieved | No change in classification | |
| Western Gotland Basin | Achieved | Achieved | No change in classification | |
| Gulf of Riga | Achieved | Failed | Worsened classification | The status has decreased to sub-GES. The certainty of the estimate is however low, as the probability of achieving the threshold was 0.51. |
| Northern Baltic Proper | Achieved | Achieved | No change in classification | |
| Gulf of Finland | Achieved | Failed | Worsened in classification | The status has decreased to sub-GES. The certainty of the estimate is however low, as the probability of achieving the threshold was 0.51. |
| Åland Sea | Achieved | Achieved | No change in classification | |
| Bothnian Sea | Achieved | Achieved | No change in classification | |
| The Quark | Achieved | Achieved | No change in classification | |
| Bothnian Bay | Achieved | Achieved | No change in classification |
5 Confidence
The confidence of the indicator results was evaluated using spatial and temporal data coverage and an estimate of the probability that the status is above the threshold derived from the distribution of the 100 000 bootstrapped BQI-values (see Assessment protocol). The overall confidence of the indicator result is generally high, although regional differences occur as there is variation in how well the spatial and temporal data covers individual assessment units. Thus, in the Eastern Gotland Basin and the Gulf of Riga overall confidence is considered to be intermediate and in the Gulf of Finland overall confidence is low.
In most assessment units, the certainty of the status classification was high (Table 6). In the Gulf of Riga and the Gulf of Finland the probability of being above the threshold was 0.51 although the indicator result was sub-GES. Consequently, the certainty of the status classification was considered low. In all other assessment units the probability of being above the threshold was consistent with the indicator results.
The spatial data coverage was considered to be high in the Kiel Bay, the Bay of Mecklenburg, the Western Gotland Basin, the Gulf of Riga, the Åland Sea, the Bothnian Sea, the Quark and the Bothnian Bay. In the Eastern Gotland Basin the spatial data coverage was considered intermediate, whereas in the Gulf of Finland and the Northern Baltic Proper there were few sampling stations with low spatial data coverage (see Figure 5). The temporal coverage of the data is considered to be high in the Eastern Gotland Basin, Gulf of Finland, Bothnian Sea and Bothnian Bay, where a majority of stations have been sampled every year in the assessment period. In other areas, data was available from all years in the assessment period, but no or few stations were sampled every and thus, the temporal coverage was considered intermediate. Sampling effort also varies, ranging between 1 and 5 samples per station and year. However, this sampling imbalance is handled via the bootstrap method used to calculate the actual indicator value used for status evaluation (see Assessment protocol).
Methodological confidence was considered to be high in assessment units where the sampling methodology and the method for defining species sensitivity values were consistent within the assessment unit. Where inconsistency in the sampling methodology or the method for defining species sensitivity values occurred, the methodological confidence was considered intermediate.
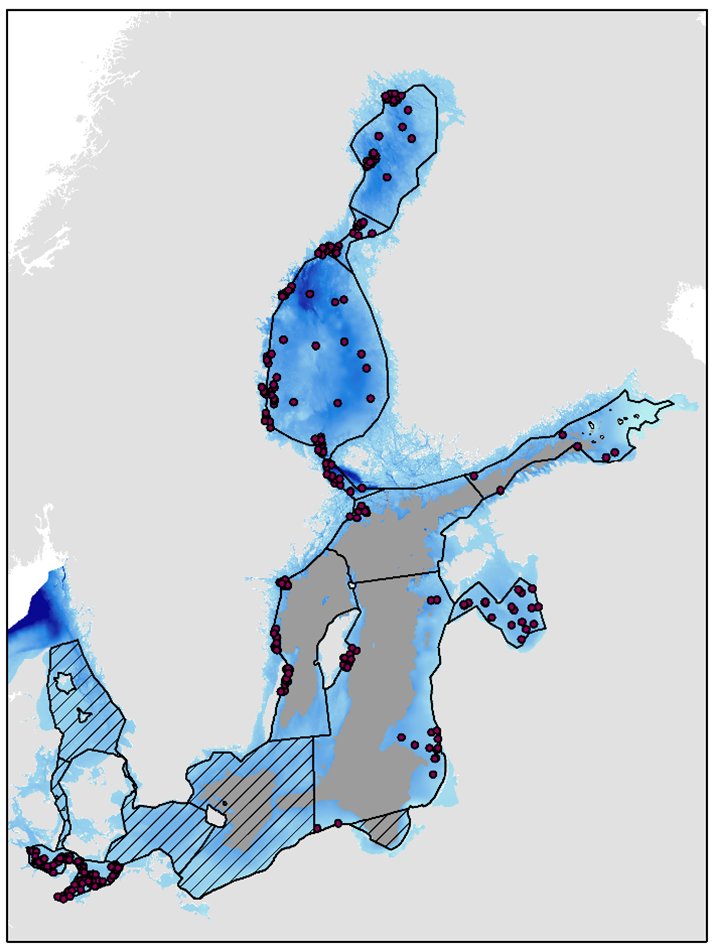
Figure 5. Soft-bottom macrofauna stations used in evaluation. Assessment units are outlined with a black line. The grey area represent below halocline depths and are only evaluated with the indicator ’Oxygen debt’. Hatched areas represent areas where no thresholds have been agreed and hence no evaluations were done.
Table 6. Confidence assessment of the indicator evaluation in individual assessment units. See text for further explanations.
| Assessment unit | Certainty of classification | Spatial coverage | Temporal coverage | Methodological confidence |
| Kiel Bay | High | High | Intermediate | High |
| Bay of Mecklenburg | High | High | Intermediate | High |
| Eastern Gotland Basin | High | Intermediate | High | Intermediate |
| Western Gotland Basin | High | High | Intermediate | High |
| Gulf of Riga | Low | High | High | Intermediate |
| Northern Baltic Proper | High | Low | Intermediate | High |
| Gulf of Finland | Low | Low | High | Intermediate |
| Åland Sea | High | High | Intermediate | High |
| Bothnian Sea | High | High | High | High |
| The Quark | High | High | Intermediate | High |
| Bothnian Bay | High | High | High | High |
6 Drivers, Activities, and Pressures
Soft-bottom macrofauna community composition is a good indicator of environmental status because the results integrate several pressures on the environment over a moderate period of time. The quality of the soft-bottom macrofauna community implies that status changes can, however, only be indirectly linked to anthropogenic pressures. The soft-bottom macrofauna community structure is affected by eutrophication (including oxygen deficiency), changes in water and sediment quality and hydrographic conditions such as salinity or temperature, as well as physical damage to the seafloor (Table 7).
Table 7. The effect of anthropogenic pressures on macrozoobenthic diversity.
| Status of diversity | Anthropogenic pressure |
| Improved | Slight eutrophication |
| Reduced | Severe eutrophication (incl. oxygen deficiency) |
| Reduced | Physical disturbance (due to abrasion, smothering, changes in siltation) |
| Reduced* | Physical loss (due to sealing or selective extraction) |
| Reduced* | Introduction of synthetic compounds (due to ship accidents or harbours) |
| Altered | Changes in the hydrological conditions (due to changes in salinity and/or temperature) |
*These may also at local scales result in altered diversity as lost or impacted areas can be evaluated at different scales (e.g. sealing will directly alter diversity in the area sealed (lost) but may reduce diversity at a broader spatial scale.
The anthropogenic pressure the indicator clearly reacts to in large areas of the Baltic Sea is eutrophication that causes hypoxia and anoxia in bottom waters (Pearson and Rosenberg 1978, Hyland et al. 2005, Norkko et al. 2006). Hypoxia has resulted in habitat destruction and the elimination of benthic macrofauna over vast areas, and has severely disrupted benthic food webs. In food-limited soft-bottom macrofauna communities, an increase in organic material input and subsequent disturbance are initially seen as large fluctuations in benthic diversity, abundance and biomass. Species composition changes as conditions deteriorate, and the advantage gained by smaller-sized and/or tolerant species results in decreasing total biomass and diversity of the soft-bottom macrofauna community as sensitive, large-sized and long-lived species disappear. At advanced stages of organic enrichment, most bottom-water oxygen is consumed by the aerobic microbial decomposition of organic material, resulting in hypoxia and anoxia and initiating the release of toxic hydrogen sulphide from the sediments. At these advanced stages of hypoxia and anoxia, soft-bottom macrofauna is eliminated and important ecosystem services are lost.
The most severe damage from the physical pressure of bottom trawling is apparent in the southern areas of the Baltic Sea where trawling intensity is higher and the soft-bottom macrofauna community is dominated by very long-lived species of clams and mussels. In other coastal areas the main physical damage of relevance to the soft-bottom macrofauna community stems from dredging activities and dumping of dredged materials. Dredging and dumping activities can change local hydrographical conditions as well as change siltation rates, especially in the short term.
Table 8 –Brief summary of relevant pressures and activities with relevance to the indicator.
| General | MSFD Annex III, Table 2a | |
| Strong link | eutrophication, seabed disturbance e.g. trawling, contamination e.g. oil spill, or aggregate extraction. | Physical
Substances, litter and energy
Biological
|
| Weak link |
7 Climate change and other factors
Climate change effects on the Baltic Sea environment are complex and may follow different patterns in the Baltic Sea region due to the extensive gradients across the region. Certain trends can be expected for example, water temperature and sea level, which are projected to rise whereas sea ice cover is projected to decrease. This will affect ecosystems and biota; for example, range shifts are expected for a number of marine species and benthic productivity will decrease. The impacts will hence affect the overall ecosystem function and influence human uses of the sea (HELCOM and Baltic Earth, 2021).
Increased freshwater inflows would bring more dissolved organic carbon to the sea, affecting benthic habitats by changes in pelagic primary production and phytoplankton sedimentation. Such a scenario would be expected in the Gulf of Bothnia region. In the Baltic proper the combined effects of warming and planned nutrient reductions will eventually lead to less carbon reaching the seafloor, reducing benthic animal biomass. In the Baltic Sea, many benthic species exist at the edge of their distribution, and even small fluctuations in temperature and salinity can impact their abundance, biomass, and spatial distribution. In concurrence with trophic cascades and eutrophication, climate change might lead to major changes in biodiversity and ecosystem functions of benthic habitats. Thus, direct climate change parameters of clear relevance to benthic habitats include: water temperature, sea ice, salinity and salt water inflows, river run off, carbonate chemistry (HELCOM and Baltic Earth, 2021).
Indirect effects such as changes in oxygen conditions, microbial processes, coastal and migratory fish, waterbirds, pelagic and demersal fish, non-indigenous species, and ecosystem functions are also likely relevant for benthic habitats. Composition, abundance, biomass and spatial distribution of benthic species and habitats, with potential loss of biodiversity and ecosystem functions may occur and the altered ecosystem function and species composition will alter networks of biodiversity, their connectivity and conservation values. Benthic soft substrate communities in large parts of the Baltic Sea have drastically changed during the past decades, with amphipods decreasing, the Baltic clam Macoma balthica increasing, and the non-indigenous polychaete Marenzelleria becoming dominant. Changes have been explained to some degree by abiotic factors such as temperature, fluctuations in salinity and oxygen, and precipitation and runoff related changes in pelagic food webs. Decreasing amount of sea ice has consequences for stratification, nutrient dynamics, primary production patterns, and hence benthic communities. Despite decreasing nutrient loads, hypoxic areas continue to prevail in the central Baltic Sea and increase in the coastal zone, causing loss of communities and ecosystem functions. Increased temperature will further worsen oxygen conditions and thus climate change impacts are critical to consider for this indicator (HELCOM and Baltic Earth, 2021).
Secondary parameters related to human use as response to climate change effects may be relevant, such as: wind farm construction, coastal defence, blue carbon storage capacity, and marine and coastal ecosystem services. Changes in human use of the Baltic Sea will also have consequences for benthic habitats resulting in instances of physical loss, disturbance, or protection/management.
8 Conclusions
The status of the indicator is good in all evaluated subbasins, except the Bay of Mecklenburg, Gulf of Riga and Gulf of Finland where the threshold value is not reached. The current evaluation spans the years 2016-2021, and is based on monitoring data reported by HELCOM Contracting Parties. The overall confidence of the indicator result is generally high, although regional differences occur as there is variation in how well the spatial and temporal data covers individual assessment units. Thus, in the Eastern Gotland Basin and the Gulf of Riga overall confidence is considered to be intermediate and in the Gulf of Finland overall confidence is considered low.
The indicator takes into account the relative proportion of sensitive and tolerant species, as well as species richness and abundance. The indicator evaluates the status of the soft-bottom macrofauna community occurring in the open sea areas of the Baltic Sea. In Northern Baltic Proper, Gulf of Finland, Eastern Gotland Basin and Western Gotland Basin only areas above the permanent halocline are evaluated. In areas below the halocline the Oxygen debt indicator should be used for the evaluation. The indicator is not used in coastal areas which are evaluated by national methods. The indicator is applicable in the open sea areas from all countries bordering the Baltic Sea, but currently it is operational only in the evaluated areas due to the lack of agreed threshold values in some assessment units.
8.1 Future work or improvements needed
For an optimal evaluation of the status of soft-bottom communities, the benthic macrofauna should be monitored in all coastal and open sea assessment units. Monitoring design should optimally take into account the habitat heterogeneity within the assessment unit to cover the spatial variation in communities. Ideally, the same methodology should be applied throughout the Baltic Sea. Improved benthic habitat maps in the future would also support better application of this indicator in downstream assessment processes (e.g. the integrated assessment of benthic habitats) and further improve confidence. Additional developments to explore temporal trends may also be of value.
9 Methodology
Evaluating the status of soft-bottom macrofauna in the open sea assessment units is done using a method based on the Benthic Quality Index (BQI), where the abundance weighted proportion of sensitive to tolerant taxa and the diversity of the community are the determining parameters. In general terms, the higher the proportion of sensitive taxa and the higher the number of different species, the better the environmental status is evaluated to be.
HELCOM Contracting Parties that are also EU Member States have developed methods for assessing the coastal areas using benthic invertebrates for the purpose of the EU Water Framework Directive (WFD). WFD Good Ecological Status (GEcS) threshold values as well as the specific index to be used, have been defined in national legislation. To avoid developing two contradictory environmental status evaluations in coastal areas (both using benthic invertebrates), the national assessments from the WFD framework in coastal areas are selected, with a common HELCOM method used for evaluating the open sea areas. In the deeper parts of some open sea areas the environmental status is evaluated with the Oxygen debt indicator (see Figure 5).
The BQI approach has been developed through several consecutive studies (Rosenberg et al. 2004, Leonardsson et al. 2009, Leonardsson et al. 2015, Leonardsson et al. 2016 and Blomqvist & Leonardsson 2016). In this core indicator the version of the index to be used is the formula presented in Leonardsson et al. (2009):
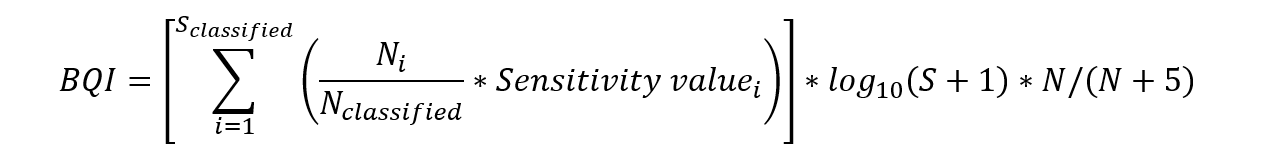
where Sclassified is the number of taxa having a sensitivity value, Ni is the number of individuals of taIon i, Nclassified is the total number of individuals of taxa having a sensitivity value, the Sensitivity valuei is the sensitivity value for tIxon i, S is the total number of taxa, and N is the total number of individuals in the sample (recalculated to 0.1 m2).
Sensitivity values used originate from two different concepts and sources; a) literature information on sensitivity to disturbance and expert knowledge (according to Leonardsson et al. 2009) are used in the Gulf of Bothnia, Åland Sea, Norther Baltic Proper and Western Gotland Basin, and b) calculated values based on taxa occurrence at different diversities according to Schiele et al. (2016) are used in the Gulf of Finland, Gulf of Riga, Eastern Gotland Basin, Bay of Mecklenburg and Kiel Bay. The sensitivity values in Leonardsson et al. (2009) are constant across the whole Baltic and the same as those used for WFD assessment in both Sweden and Finland. Sensitivity values calculated according to Schiele et al. (2016) are different for different salinity/depth/gear subsets. Salinities for the sampling stations were retrieved using the modelled EUSeaMap values in order to have a set salinity for the sampling stations and avoid stations being assigned different subset factors based on the observed salinity at sampling. In total 19 different subsets are identified by Schiele et al. (2016) but only eight of these are present in the assessment units where this concept is currently used. In each of these assessment units more than one subset is present. Since each subset will have a different range of sensitivity values separate calculations have to be performed for each subset and these separate status evaluations are subsequently merged to give a final evaluation value for the specific assessment unit.
9.1 Scale of assessment
The indicator is calculated for HELCOM assessment unit scale 4 open sea assessment units. There are 17 open sea assessment units defined in the HELCOM Monitoring and Assessment Strategy Annex 4. The units are delineated based on the 1 nautical mile boundary from the coastal baseline.
The indicator evaluates the open sea areas of the Baltic Sea though the areas below the permanent halocline are not evaluated with this indicator (see Figure 1).
9.2 Methodology applied
Assessment calculations
In the Gulf of Finland, Gulf of Riga, Eastern Gotland Basin, Bay of Mecklenburg and Kiel Bay assessment units more than one set of sensitivity values were used in the calculation of BQI, i.e., one for each Schiele subset. To make the BQI values comparable across the Schiele subsets, normalization to a common scale between 0 and 1 was done, with the threshold value scaled to 0.5 and setting the maximum observed BQI value in the subset to 1. In the assessment units where only one set of sensitivity values were used (Bothnian Bay, The Quark, Bothnian Sea, Åland Sea, Northern Baltic Proper and Western Gotland Basin) thus no normalization was needed for the BQI values.
In order to account for spatial, temporal and sample replicate imbalance a bootstrap procedure was used to estimate the 20th percentile to be compared against the threshold value. The 20th percentile is used as a precautionary or “fail-safe” approach (Carstensen 2007, Leonardsson et al. 2009) placing results of high uncertainty into lower status categories. Spatial imbalance with several stations concentrated to smaller parts of the evaluation area is overcome by defining geographic sub-areas. Spatial imbalance often arises from different monitoring designs used by different HELCOM Contracting Parties, e.g. the cluster design in Sweden or the single station design in Finland (e.g. Figure 6).
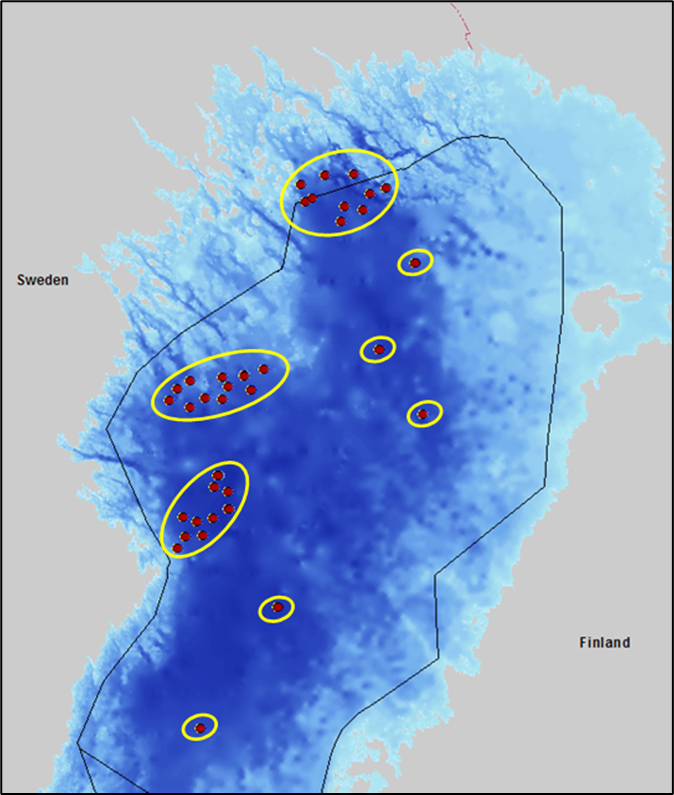
Figure 6. Example of geographic sub-areas used to overcome spatial imbalance in the bootstrap process. Red dots represent sampling stations and yellow circles indicate geographic subareas in the Bothnian Bay assessment unit.
The bootstrap process for one assessment unit and one assessment period follows these steps:
- Random selection of one geographic subarea
- Random selection of one station from this subarea
- Random selection of one grab sample from this station, irrespective of year and sample number, and store its BQI-value
- Repeat steps 1 to 3 as many times as there are stations in the assessment unit
- Calculate the mean of the stored BQI-values and store this mean BQI-value
- Repeat above steps 100 000 times
- Calculate the 20th percentile of the stored 100 000 mean BQI-values
The 20th percentile from step 7 is the indicator value to be compared with the threshold value of the specific assessment unit.
9.3 Monitoring and reporting requirements
Monitoring methodology
Monitoring of soft-bottom macrofauna is described in general terms in the HELCOM Monitoring Manual in the Programme Topic Benthic community species distribution and abundance. Monitoring specifically on soft-bottom macrofauna communities is further described in the sub-programme: Softbottom fauna. The Monitoring Concepts Table summarizes ongoing monitoring activities.
Monitoring guidelines describing the sampling strategy are to be included in the HELCOM Monitoring Manual. The guidelines are currently under development and will be included once agreement has been reached.
Current monitoring
The monitoring activities relevant to this indicator, as currently carried out by HELCOM Contracting Parties, are described in the HELCOM Monitoring Manual
Sub-programme:
The Monitoring Concepts Table lists the Contracting Parties currently monitoring soft-bottom macrofauna.
10 Data
The data and resulting data products (e.g. tables, figures and maps) available on the indicator web page can be used freely given that it is used appropriately and the source is cited.
Result: State of the soft-bottom macrofauna community
Data: State of the soft-bottom macrofauna community
The snapshot dataset on macrofauna includes data from EE, FI, DE, LV, LT, PL, SE.
Data was extracted from the HELCOM COMBINE database, hosted by ICES. The extracted dataset was supplemented with additional data from Germany.
Offshore waters: Monitoring started in some places in 1964. The current status evaluation is based on data from 2016-2021. Monitoring is on-going and contracting parties report data annually to the HELCOM COMBINE database.
11 Contributors
Co-Lead Country representatives: Henrik Nygård, Mats Blomqvist
Other acknowledged contributors: HELCOM Expert Network on Benthic habitats (EN-BENTHIC)
HELCOM Secretariat: Jannica Haldin, Owen Rowe
12 Archive
This version of the HELCOM core indicator report was published in April 2023:
The current version of this indicator (including as a PDF) can be found on the HELCOM indicator web page.
Earlier versions of this indicator report are available:
State of the soft-bottom macrofauna community HELCOM core indicator report 2018 (pdf).
HOLAS II component – Core indicator report – web-based version July 2017 (pdf)
13 References
Andersin, A.-B., J. Lassig, L. Parkkonen & H. Sandler (1978). The decline of macrofauna in the deeper parts of Baltic Proper and Gulf of Finland. Kieler Meeresforschungen 4:23–52.
Blomqvist, M., Leonardsson, K. (2016). A probability based index for assessment of benthic invertebrates in the Baltic Sea. Deliverable D3.1-4, WATERS Report no. 2016:3 Havsmiljöinstitutet, Sweden.
Bonsdorff, E., Pearson, T.H. (1999). Variations in the sublittoral macrozoobenthos of the Baltic Sea along environmental gradients: a functional-group approach. Aust. J. Ecol. 24, 312–326.
Carletti, A., Heiskanen, A.-S. (2009). Water Framework Directive Intercalibration Technical Report. Part 3: Coastal and Transitional Waters. JRC Scientific and Technical Reports, EUR 23838 EN/3: 240 pp. Doi: 10.2788/19561
Carstensen, J. (2007). Statistical principles for ecological status classification of Water Framework Directive monitoring data. Marine Pollution Bulletin 55, 3–15.
Elmgren, R. (1989). Man’s impact on the ecosystem of the Baltic Sea: Energy flows today and at the turn of the century. – Ambio 18: 326-332.
Gogina M, Nygård H, Blomqvist M, Daunys D, Josefson AB, Kotta J, Maximov A, Warzocha J, Yermakov V, Gräwe U, Zettler ML (2016) The Baltic Sea scale inventory of benthic faunal communities. ICES Journal of Marine Science 73:1196-1213 (doi: 10.1093/icesjms/fsv265).
Hyland, J., L. Balthis, I. Karakassis, P. Magni, A. Petrov, J. Shine, O. Vestergaard, and R. Warwick. (2005). Organic carbon content of sediments as an indicator of stress in the marine benthos. Marine Ecology Progress Series 295:91–103.
HELCOM and Baltic Earth, 2021. Climate Change in the Baltic Sea 2021 Fact Sheet.
Leonardsson, K., Blomqvist, M., Rosenberg, R. (2009). Theoretical and practical aspects on benthic quality assessment according to the EU-Water Framework Directive – examples from Swedish waters. Marine Pollution Bulletin 58, 1286–1296.
Leonardsson, K., Blomqvist, M., Magnusson, M., Wikström, A., Rosenberg, R. (2015). Calculation of species sensitivity values and their precision in marine benthic faunal quality indices. Mar. Pollut. Bull. 93, 94-102.
Leonardsson, K., Blomqvist, M., Rosenberg, R. (2016). Reducing spatial variation in environmental assessment of marine benthic fauna. Mar. Pollut. Bull. Doi: 10.1016/j.marpolbul.2016.01.050.
Norkko, A., R. Rosenberg, S. F. Thrush, and R. B. Whitlatch. (2006). Scale- and intensity-dependent disturbance determines the magnitude of opportunistic response. Journal of Experimental Marine Biology and Ecology 330:195–207.
Pearson, T. H., and R. Rosenberg. (1978). Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: an Annual Review 16:229–311.
Perus J, Bonsdorff E, Bäck S, Lax HG, Villnäs A, Westberg V (2007): Zoobenthos as indicators of ecological status in coastal brackish waters: a comparative study from the Baltic Sea. Ambio 36, 250–256.
Rosenberg, R., Blomqvist, M., Nilsson, H.C., Cederwall, H., Dimming, A., (2004). Marine quality assessment by use of benthic species-abundance distributions: a proposed new protocol within the European Union Water Framework Directive. Marine Pollution Bulletin 49, 728–739.
Rumohr H., Bonsdorff E., Pearson T. H. (1996). Zoobenthic succession in Baltic sedimentary habitats. Arch. Fish. Mar.Res. 4, 179–214.
Schiele KS, Darr A, Zettler ML, Berg T, Blomqvist M, Daunys D, Jermakovs V, Korpinen S, Kotta J, Nygård H, von Weber M, Voss J, Warzocha J. (2016). Rating species sensitivity throughout gradient systems – a consistent approach for the Baltic Sea. Ecological indicators 61:447-455 (doi: 10.1016/j.ecolind.2015.09.046).
Villnäs, A. & Norkko, A. (2011). Benthic diversity gradients and shifting baselines: implications for assessing environmental status. Ecological Applications 21: 2172–2186.
14 Other relevant resources
No additional components are provided in this report.